おすすめの製品
アプリケーション
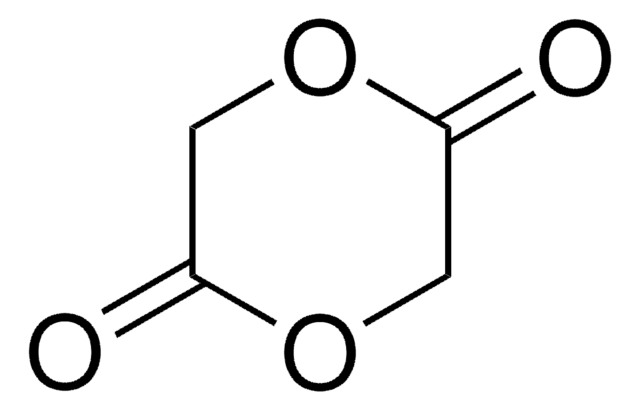
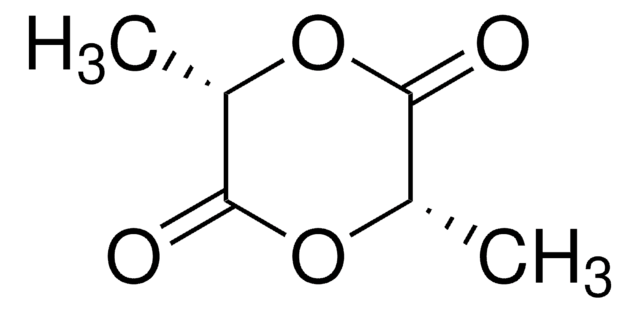
3-Methylglycolide (MG) is a six-member lactone consisting of one lactyl unit (L) and one glycolyl unit (G), it is used for the synthesis of poly (lactic-co-glycolic acid) (PLGA) polymer. Polymerization of MG follows the same mechanism as that of glycolide (GA) and Lactide (LA), and the resulting polymers possess the exact alternative sequence of glycolide (GA) and Lactide (LA). PLGA based polymers made from MG perfectly avoid the drawback of structure with long glycolic blocks, exhibit excellent solubility in common organic solvents, such as acetonitrile, acetone, dioxane, DCM and THF, thus solve the long-time headache of insolubility and discoloration issues of PLGA based polymers, providing great convenience for drug delivery researches and applications.
Poly(lactic-co-glycolic acid) (PLGA) is a biocompatible and biodegradable polymer that has been approved by the FDA for biomedical and pharmaceutical applications. PLGA based polymers can be synthesized from copolymerization of glycolide (GA) and lactide (LA), and PLGAs that comprise up to a 1:1 ratio of lactic to glycolic units are of practical interest. However, the copolymerization of glycolide (GA) and Lactide (LA) typically results in broad composition ranges and a random block nature because of the much higher reactivity of GA and the drastic polymerization conditions. Thus, simple use of equimolar charges of GA and LA results in polymers containing longer glycolic blocks. This adversely affects the solubility and PDI of the copolymer. Melt copolymerization of GA and LA has often been used to prepare PLGA with high glycolic content. Under these conditions, in situ transesterification of the polymer both randomizes the sequence and broadens the distribution, as well as significant discoloration of the resulting PLGA copolymer
Poly(lactic-co-glycolic acid) (PLGA) is a biocompatible and biodegradable polymer that has been approved by the FDA for biomedical and pharmaceutical applications. PLGA based polymers can be synthesized from copolymerization of glycolide (GA) and lactide (LA), and PLGAs that comprise up to a 1:1 ratio of lactic to glycolic units are of practical interest. However, the copolymerization of glycolide (GA) and Lactide (LA) typically results in broad composition ranges and a random block nature because of the much higher reactivity of GA and the drastic polymerization conditions. Thus, simple use of equimolar charges of GA and LA results in polymers containing longer glycolic blocks. This adversely affects the solubility and PDI of the copolymer. Melt copolymerization of GA and LA has often been used to prepare PLGA with high glycolic content. Under these conditions, in situ transesterification of the polymer both randomizes the sequence and broadens the distribution, as well as significant discoloration of the resulting PLGA copolymer
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Eye Irrit. 2
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
919098-VAR:
919098-1G:
919098-BULK:
最新バージョンのいずれかを選択してください:
Synthesis of O-(2′-Bromopropionyl)glycolic Acid and Its Polymerization: Synthesis of an Alternating Lactic and Glycolic Acid Copolymer.
Rebert WN
Macromolecules, 27, 5533-5535 (1994)
Lin Yu et al.
Biomacromolecules, 12(4), 1290-1297 (2011-03-03)
This paper reports the influence of sequence structures of block copolymers composed of poly(lactic acid-co-glycolic acid) (PLGA) and poly(ethylene glycol) (PEG) on their thermogelling aqueous behaviors. A series of thermogelling PLGA-PEG-PLGA triblock copolymers with similar chemical compositions and block lengths
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)