おすすめの製品
詳細
Drug loading screening kit, for synthesis of PEGylated PLGA nanoparticles
Kit components :
PEGPLGA-50L(912808-500mg)
PEGPLGA-75L(913049-500mg)
PEGPLGA-50H (915955-500mg)
PEGPLGA-75H (915718-500mg)
Stabilizer-Nano (907766-5g)
品質水準
アプリケーション
advanced drug delivery
保管温度
2-8°C
詳細
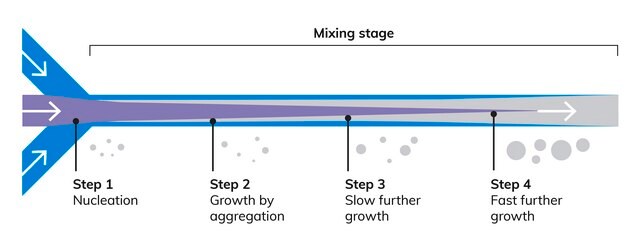
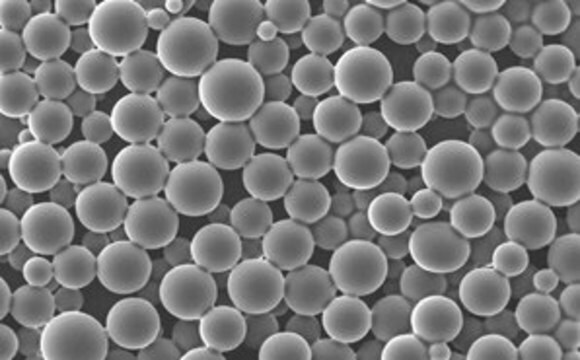
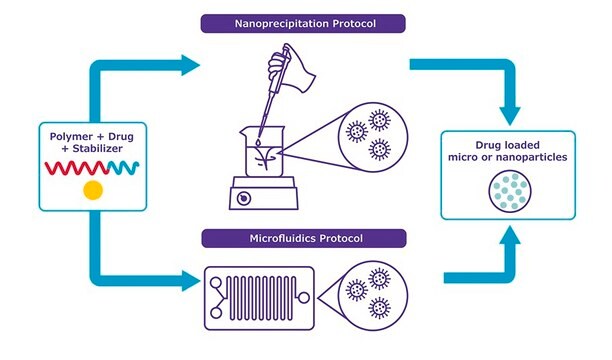
NanoFabTx™ PEG-PLGA drug loading screening kit is a ready-to-use nanoformulation kit for the synthesis of PEGylated PLGA nanoparticles 50 nm to 350 nm in size. This kit provides properly selected PEGylated PLGAs, stabilizer, and formulation protocols for synthesis by nanoprecipitation and microfluidic methods. Four different PEGylated PLGAs are included in this kit, allowing rapid screening of optimal materials for enhancing drug loading. Protocols for two different particle synthesis methods are included.
- A Nanoprecipitation protocol to prepare drug-encapsulated nanoparticles in standard laboratory glassware.
- A Microfluidics protocol using commercial platforms or syringe pumps.
アプリケーション
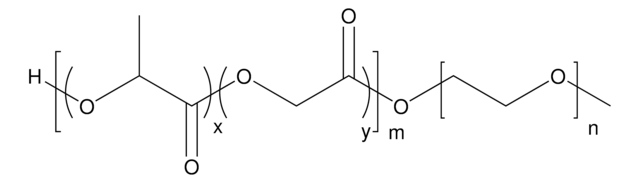
NanoFabTx™ nanoformulation reagent kits enable users to encapsulate a wide variety of therapeutic drug molecules in PEGylated PLGA nanocarriers for targeted or sustained drug delivery without the need for lengthy trial-and-error optimization. PEGylated PLGAs are well suited towards applications involving sustained release because PEGylation has been shown to improve the circulation half-life of polymeric nanocarriers by altering the solubility and shielding the nanocarrier from enzymes and antibodies that may induce degradation, secretion, and clearance. Because poly(lactic-co-glycolic acid) (PLGA) is a biocompatible and biodegradable polymer approved by the FDA for biomedical and pharmaceutical applications, PLGA-based nanoparticles synthesized with the NanoFabTx™ kits are suitable for biomedical research applications such as oncology, immuno-oncology, gene delivery, and vaccine delivery.
特徴および利点
- Requires minimal laboratory setup
- Optimized protocols with step-by-step instructions for either nanoprecipitation or microfluidics-based synthesis
- Yields specifically sized, low polydispersity biodegradable, PEGylated PLGA nanoparticles from 50 nm to 350 nm in size
- Maximizes the encapsulation of hydrophobic drugs
- Four different PEGylated PLGAs are included
法的情報
NANOFABTX is a trademark of Sigma-Aldrich Co. LLC
関連製品
製品番号
詳細
価格
保管分類コード
10 - Combustible liquids
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
917796-1KT:
917796-BULK:
917796-VAR:
917796-1EA:
最新バージョンのいずれかを選択してください:
Susanne Schöttler et al.
Nature nanotechnology, 11(4), 372-377 (2016-02-16)
The current gold standard to reduce non-specific cellular uptake of drug delivery vehicles is by covalent attachment of poly(ethylene glycol) (PEG). It is thought that PEG can reduce protein adsorption and thereby confer a stealth effect. Here, we show that
Hassan A Almoustafa et al.
International journal of pharmaceutics, 533(1), 275-284 (2017-09-26)
Nanoprecipitation is a simple and increasingly trending method for nanoparticles preparation. The self-assembly feature of poly (ethylene glycol)-poly (lactide-co-glycolic acid) (PEG-PLGA) amphiphilic copolymer into a nanoparticle and its versatile structure makes nanoprecipitation one of the best methods for its preparation.
Y Li et al.
Journal of controlled release : official journal of the Controlled Release Society, 71(2), 203-211 (2001-03-29)
The aim of the present work was to assess the merits of PEGylated poly(lactic-co-glycolic acid) (PEG-PLGA) nanoparticles as protein and peptide drugs (PPD) carriers. PEG-PLGA copolymer, which could be used to prepare the stealth nanoparticles or long-circulating nanoparticles, was synthesized
L Martin-Banderas et al.
Mini reviews in medicinal chemistry, 13(1), 58-69 (2012-09-15)
This article presents the potential of PLGA nanoparticles for the oral administration of drugs. Different strategies are used to improve oral absorption of these nanoparticles. These strategies are based on modification of nanoparticle surface properties. They can be achieved either
Which polymers can make nanoparticulate drug carriers long-circulating?
Torchilin V P, et al.
Advanced Drug Delivery Reviews, 16, 141-155 (1995)
Global Trade Item Number
| カタログ番号 | GTIN |
|---|---|
| 917796-1EA | 4065265015202 |
| 917796-1KT | 4065266037289 |
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)