おすすめの製品
品質水準
アッセイ
≥95%
フォーム
powder or crystals
反応適合性
reagent type: catalyst
環境により配慮した代替製品の特徴
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
環境により配慮した代替製品カテゴリ
, Aligned
保管温度
−20°C
SMILES記法
[H]C1([H])C([H])([H])O[C@@](C([H])([H])O[Si](C([H])([H])[H])(C([H])([H])[H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])([H])[C@](O[H])([H])[C@]1([H])O[H]
InChI
1S/C12H26O4Si/c1-12(2,3)17(4,5)16-8-10-11(14)9(13)6-7-15-10/h9-11,13-14H,6-8H2,1-5H3/t9-,10-,11+/m1/s1
InChI Key
KKFGQPZYDWCKRC-MXWKQRLJSA-N
詳細
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information.
アプリケーション
6-Tertbutyldimethylsilyl-1,2-dihydroglucal (TBS-DHG) was reported by the Morken Lab to be an effective carbohydrate-derived catalyst for enantioselective diboration of alkenes. Related capabilities were observed with the dihydrorhamnal (DHR) catalyst (901237). TBS-DHG has also been used in ruthenium(0)catalyzed transfer hydrogenation cycloadditions.
その他情報
- Carbohydrate-Catalyzed Enantioselective Alkene Diboration:Enhanced Reactivity of 1,2-Bonded Diboron Complexes
- Diols, α-Ketols, and Diones as 22π Components in [2+2+2] Cycloadditions of 1,6-Diynes via Ruthenium(0)-Catalyzed Transfer Hydrogenation
- Carbohydrate/DBU Cocatalyzed Alkene Diboration: Mechanistic Insight Provides Enhanced Catalytic Efficiency and Substrate Scope
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
Zachary A Kasun et al.
Chemical communications (Cambridge, England), 50(56), 7545-7547 (2014-06-04)
A new method for the ring expansion of cyclic diols is described. Using improved conditions for the ruthenium(0) catalyzed cycloaddition of cyclic 1,2-diols with 1,3-dienes, fused [n.4.0] bicycles (n = 3-6) are formed, which upon exposure to iodosobenzene diacetate engage
Hiroki Sato et al.
Journal of the American Chemical Society, 138(50), 16244-16247 (2016-12-10)
The first use of vicinal diols, ketols, or diones as 2
Lichao Fang et al.
Journal of the American Chemical Society, 138(8), 2508-2511 (2016-02-09)
Catalytic enantioselective diboration of alkenes is accomplished with readily available carbohydrate-derived catalysts. Mechanistic experiments suggest the intermediacy of 1,2-bonded diboronates.
Lu Yan et al.
Journal of the American Chemical Society, 140(10), 3663-3673 (2018-02-15)
A mechanistic investigation of the carbohydrate/DBU cocatalyzed enantioselective diboration of alkenes is presented. These studies provide an understanding of the origin of stereoselectivity and also reveal a strategy for enhancing reactivity and broadening the substrate scope.
資料
Enantioselective alkene diboration is a valuable strategy for transforming unsaturated hydrocarbons into useful chiral building blocks.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)
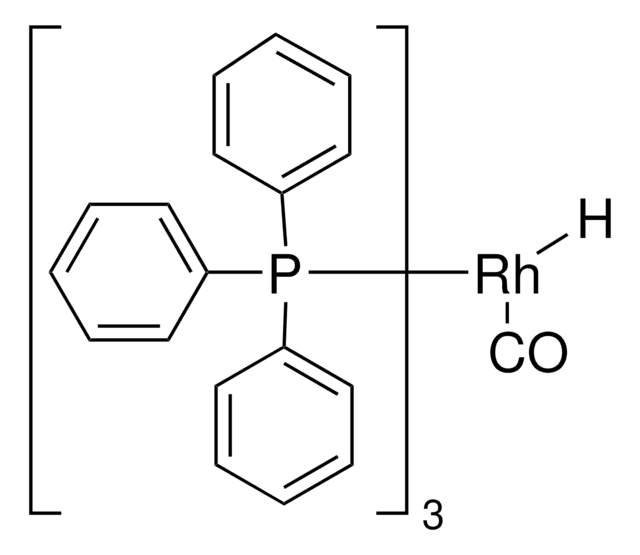
![1,8-ジアザビシクロ[5.4.0]ウンデカ-7-エン 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
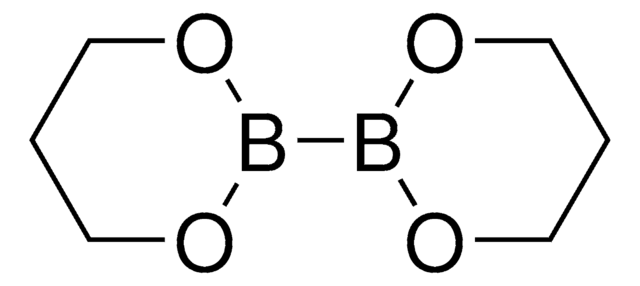
![ジクロロ[9,9-ジメチル-4,5-ビス(ジフェニルホスフィノ)キサンテン]パラジウム(II) 95%](/deepweb/assets/sigmaaldrich/product/structures/374/597/f7932c5b-0448-498b-8254-f8ce1b9a4612/640/f7932c5b-0448-498b-8254-f8ce1b9a4612.png)