おすすめの製品
品質水準
製品種目
ReagentPlus®
アッセイ
99%
フォーム
powder
光学活性
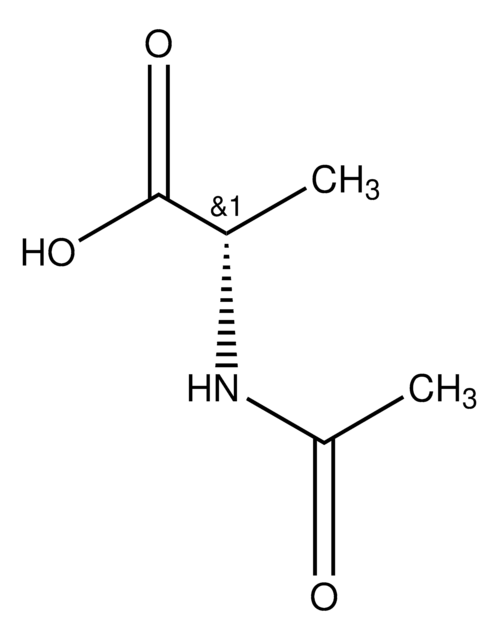
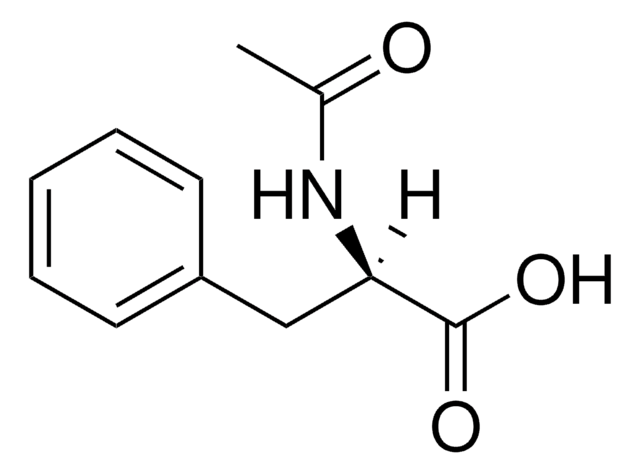
[α]22/D +40.0°, c = 1 in methanol
反応適合性
reaction type: C-H Activation
reaction type: solution phase peptide synthesis
reagent type: ligand
reaction type: Peptide Synthesis
mp
171-173 °C (lit.)
アプリケーション
peptide synthesis
官能基
amine
carboxylic acid
SMILES記法
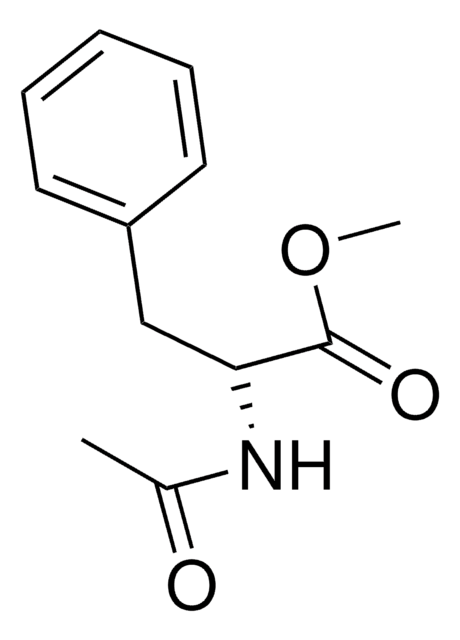
CC(=O)N[C@@H](Cc1ccccc1)C(O)=O
InChI
1S/C11H13NO3/c1-8(13)12-10(11(14)15)7-9-5-3-2-4-6-9/h2-6,10H,7H2,1H3,(H,12,13)(H,14,15)/t10-/m0/s1
InChI Key
CBQJSKKFNMDLON-JTQLQIEISA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
N-Acetyl-L-phenylalanine is an acetyl analog of L-phenylalanine. It is widely used as a reactant to synthesize methyl or ethyl esters of N-acetyl-L-phenylalanine, which are employed as versatile building blocks in peptide synthesis.
アプリケーション
N-Acetyl-L-phenylalanine can be used as a reactant to synthesize:
- N-acetyl phenylalanine methyl ester by esterification reaction with methanol using Mukaiyama′s reagent.
- Acetylaminocyclohexane propanoic acid by rhodium-catalyzed hydrogenation reaction.
法的情報
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
857459-VAR:
857459-5G:
857459-1G:
857459-BULK:
この製品を見ている人はこちらもチェック
Surfactant-protease complex as a novel biocatalyst for peptide synthesis in hydrophilic organic solvents
Okazaki S, et al.
Enzyme and Microbial Technology, 26(2-4), 159-164 (2000)
Corinna Neuber et al.
Analytical chemistry, 86(18), 9065-9073 (2014-08-20)
Sphingosine 1-phosphate (S1P), a bioactive lipid involved in various physiological processes, can be irreversibly degraded by the membrane-bound S1P lyase (S1PL) yielding (2E)-hexadecenal and phosphoethanolamine. It is discussed that (2E)-hexadecenal is further oxidized to (2E)-hexadecenoic acid by the long-chain fatty
K Y Xu
Biochemistry, 28(17), 6894-6899 (1989-08-22)
A combination of competitive labeling with [3H]acetic anhydride [Kaplan, H., Stevenson, K. J., & Hartley, B. S. (1971) Biochem. J. 124, 289-299] and immunoaffinity chromatography is described that permits the assignment of the acid dissociation constant and the absolute nucleophilicity
E Jellum et al.
Scandinavian journal of clinical and laboratory investigation. Supplementum, 184, 21-26 (1986-01-01)
Urinary organic acid profiles of patients with Maple Syrup Urine Disease (MSUD), hereditary tyrosinemia and phenylketonuria (PKU) have been studied by means of capillary GC-MS-computer technique. In addition to the characteristic metabolites of these disorders, increased amounts of N-acetylleucine, N-acetylisoleucine
Edward A Lemke
Methods in molecular biology (Clifton, N.J.), 751, 3-15 (2011-06-16)
Studies of protein structure and function using single-molecule fluorescence resonance energy transfer (smFRET) benefit dramatically from the ability to site-specifically label proteins with small fluorescent dyes. Genetically encoding the unnatural amino acid (UAA) p-acetylphenylalanine is an efficient way to introduce
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)