すべての画像(1)
About This Item
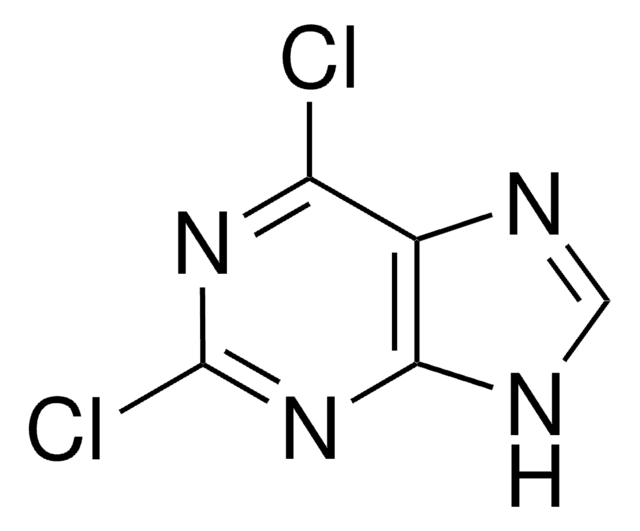
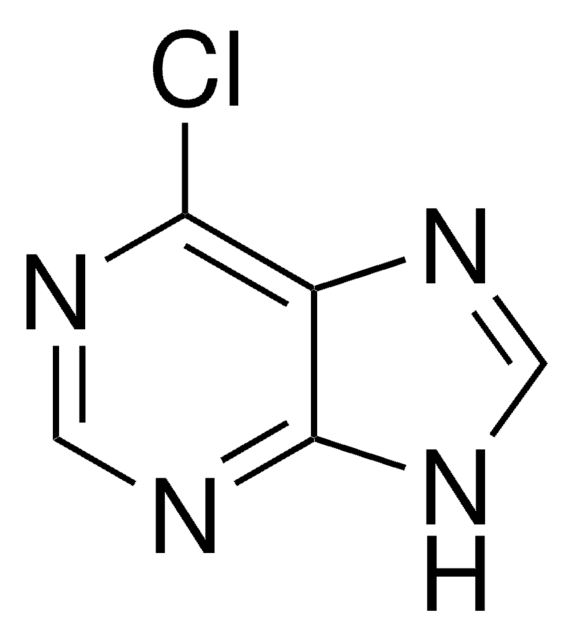
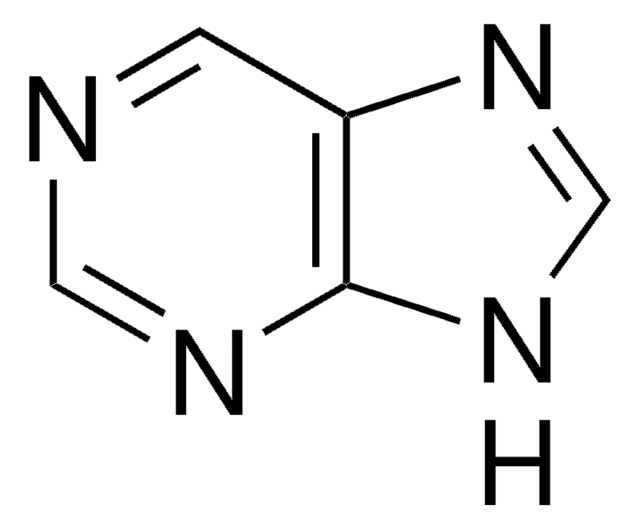
実験式(ヒル表記法):
C5H4ClN5
CAS番号:
分子量:
169.57
MDL番号:
UNSPSCコード:
12352100
PubChem Substance ID:
NACRES:
NA.22
おすすめの製品
品質水準
アッセイ
96%
フォーム
solid
mp
384-389 °C
官能基
chloro
SMILES記法
Nc1nc(Cl)nc2nc[nH]c12
InChI
1S/C5H4ClN5/c6-5-10-3(7)2-4(11-5)9-1-8-2/h1H,(H3,7,8,9,10,11)
InChI Key
HBJGQJWNMZDFKL-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
717592-VAR:
717592-500MG:
717592-BULK:
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
Kiran Kumar Akula et al.
Epilepsy research, 78(1), 60-70 (2007-12-07)
Multiple lines of investigations have explored the role of cyclooxygenases (COX) in epilepsy and related neuropsychiatric disorders. Cyclooxygenase particularly, COX-2 expression was found to increase in brain during seizure paradigms. The present study was carried out to investigate the effect
P Hentosh et al.
Molecular carcinogenesis, 13(4), 245-253 (1995-08-01)
2-Chloro-2'-deoxyadenosine (cladribine), an analog of deoxyadenosine, is an important new drug for the treatment of hairy cell leukemia and other forms of adult and pediatric leukemia. By a gel-shift binding assay, we identified an activity in HeLa nuclear extracts that
P Hentosh et al.
Molecular pharmacology, 45(5), 955-961 (1994-05-01)
2'-Chloro-2'-deoxyadenosine triphosphate (cladribine), a purine nucleotide analog and potent antileukemic agent, was enzymatically incorporated into 98-base oligomers in place of dATP to investigate the molecular consequences of 2-chloroadenine (CIAde) in DNA. We have used the resultant oligomers as templates for
P Hentosh et al.
Analytical biochemistry, 201(2), 277-281 (1992-03-01)
By utilization of polymerase chain reaction techniques, single-stranded DNA of defined length and sequence containing a purine analog, 2-chloroadenine, in place of adenine was synthesized. This was accomplished by a combination of standard polymerase chain amplification reactions with Thermus aquaticus
Synnöve Lindemalm et al.
Cancer letters, 210(2), 171-177 (2004-06-09)
The nucleoside analog 2-chlorodeoxyadenosine (Cladribine, CdA) is used in the treatment of patients with several hematological malignancies. After administration of CdA, the major catabolite measured in plasma and urine is 2-chloroadenine (CAde). This study was performed to determine the pharmacokinetics
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)