すべての画像(2)
About This Item
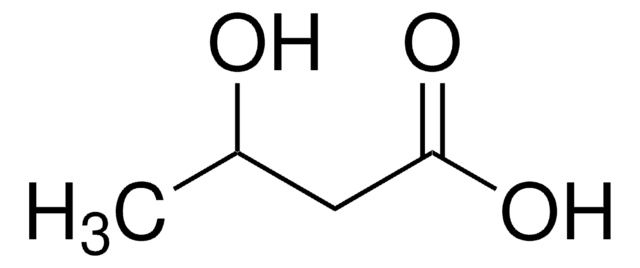
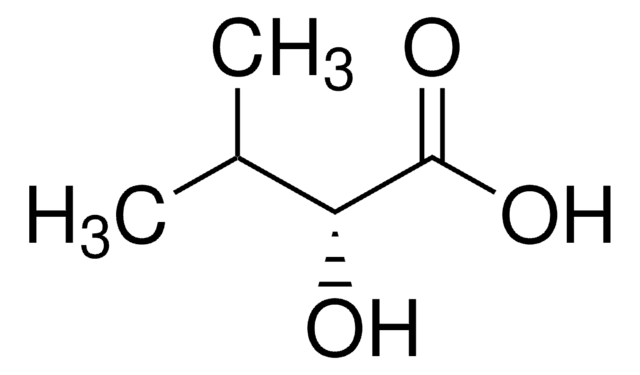
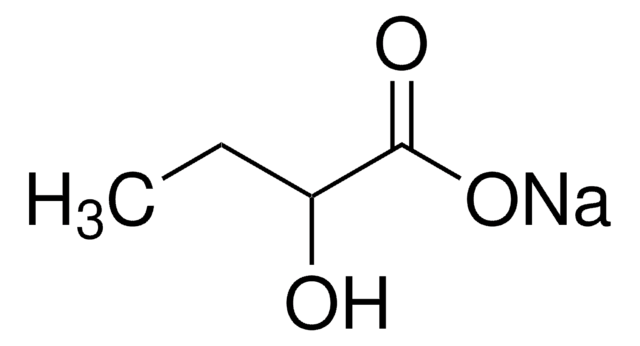
実験式(ヒル表記法):
C4H8O3
CAS番号:
分子量:
104.10
Beilstein:
1720939
MDL番号:
UNSPSCコード:
51113400
PubChem Substance ID:
NACRES:
NA.22
おすすめの製品
アッセイ
≥97.0% (T)
フォーム
solid
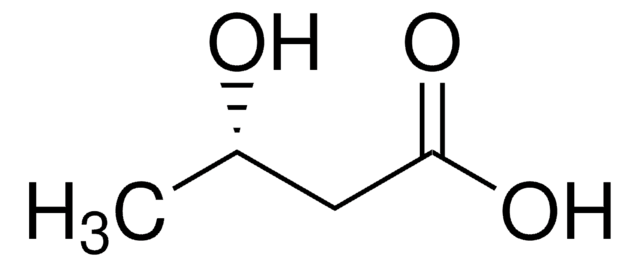
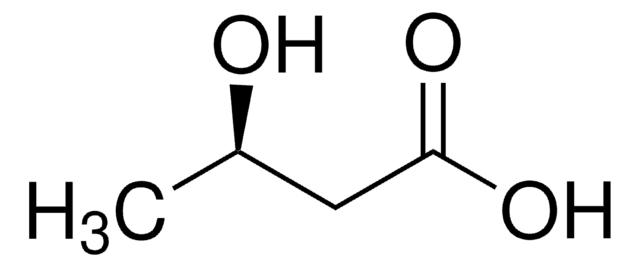
光学純度
enantiomeric ratio: ≥99:1 (GC)
mp
50-54 °C
官能基
carboxylic acid
hydroxyl
保管温度
2-8°C
SMILES記法
CC[C@@H](O)C(O)=O
InChI
1S/C4H8O3/c1-2-3(5)4(6)7/h3,5H,2H2,1H3,(H,6,7)/t3-/m1/s1
InChI Key
AFENDNXGAFYKQO-GSVOUGTGSA-N
その他情報
キラルなビルディングブロック。
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
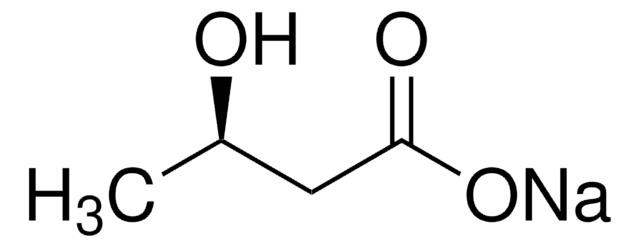
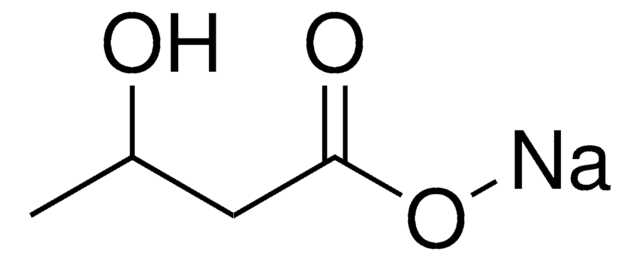
この製品を見ている人はこちらもチェック
K.J. Hale et al.
Tetrahedron Letters, 36, 6965-6965 (1995)
M N Romanelli et al.
Chirality, 8(8), 579-584 (1996-01-01)
The enantiomers of 3-alpha-tropyl 2-(phenylthio)butyrate (SM32, 1) were prepared by chiral synthesis and tested for analgesic, cognition-enhancing, and ACh-releasing properties. They show enantioselectivity in some of the tests, the eutomer being related in configuration to R-(+)-hyoscyamine.
M N Romanelli et al.
Chirality, 8(3), 225-233 (1996-01-01)
The enantiomers of two alpha-tropanyl esters, SM21 (1) and PG9 (2), derived from (+)-R-hyoscyamine, that act by increasing the central cholinergic tone, were obtained by esterification after resolution of the corresponding racemic acids [(-)-S-1, (-)-R-2 and (+)-S-2] and by stereospecific
Si Jae Park et al.
Applied microbiology and biotechnology, 93(1), 273-283 (2011-08-16)
We have previously reported in vivo biosynthesis of polylactic acid (PLA) and poly(3-hydroxybutyrate-co-lactate) [P(3HB-co-LA)] employing metabolically engineered Escherichia coli strains by the introduction of evolved Clostridium propionicum propionyl-CoA transferase (Pct(Cp)) and Pseudomonas sp. MBEL 6-19 polyhydroxyalkanoate (PHA) synthase 1 (PhaC1(Ps6-19)).
Philip J Saylor et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 18(13), 3677-3685 (2012-05-17)
Androgen deprivation therapy (ADT) for prostate cancer causes an increase in fasting insulin and adverse changes in body composition and serum lipid profile. It is unknown what other metabolic alterations are caused by ADT. To better characterize the metabolic effects
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)

![[(3R)-3-Hydroxybutyryl]-L-carnitine analytical standard](/deepweb/assets/sigmaaldrich/product/structures/658/500/ff9570f8-a346-4077-9983-d0e67400bf47/640/ff9570f8-a346-4077-9983-d0e67400bf47.png)
![[(3R)-3-Hydroxyoctadecanoyl]-L-carnitine analytical standard](/deepweb/assets/sigmaaldrich/product/structures/195/646/9c581614-9449-4107-a05e-7c573a907483/640/9c581614-9449-4107-a05e-7c573a907483.png)