すべての画像(1)
About This Item
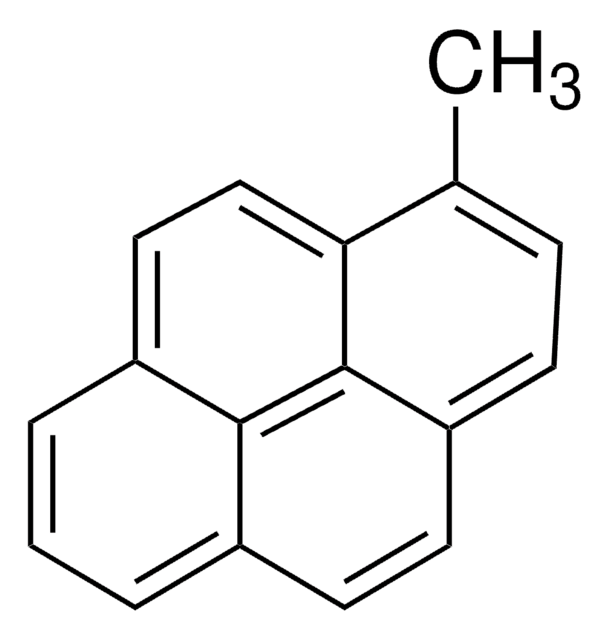
実験式(ヒル表記法):
C18H12O
CAS番号:
分子量:
244.29
MDL番号:
UNSPSCコード:
12352100
PubChem Substance ID:
NACRES:
NA.22
おすすめの製品
アッセイ
97%
フォーム
solid
mp
86-89 °C (lit.)
官能基
ketone
SMILES記法
CC(=O)c1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C18H12O/c1-11(19)15-9-7-14-6-5-12-3-2-4-13-8-10-16(15)18(14)17(12)13/h2-10H,1H3
InChI Key
KCIJNJVCFPSUBQ-UHFFFAOYSA-N
詳細
1-Acetylpyrene is a pyrene derivative. Its synthesis has been reported. Its phytophysical properties have been studied using absolute fluorescence quantum yield measurement and time-dependent density functional theory (TD-DFT) calculations. Its ability to interact with human cytochromes P450 2A13, 2A6, and 1B1 and enzyme inhibition has been reported. Its function as an environment-sensitive fluorophore has been investigated.
アプリケーション
1-Acetylpyrene is suitable for use in a comparative study on the photoinitiating efficiency of pyrene, 1-acetylpyrene and 1-(bromoacetyl)pyrene for copolymerization of styrene with acrylonitrile. It may be used in the following studies:
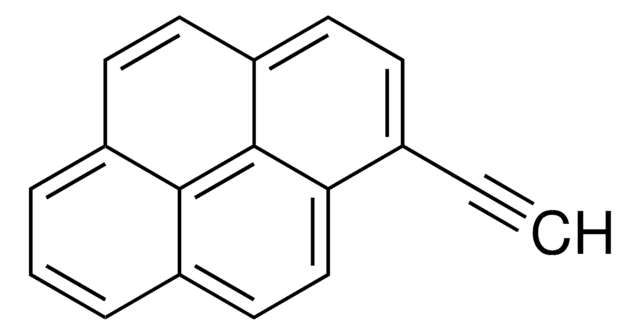
- As a starting material in the synthesis of ethynlypyrene. 1-(1-chlorovinyl)pyrene was also isolated during this reaction.
- As a starting material in the synthesis of substituted pyrene derivatives incorporated heterocyclic and sugar moieties.
- Synthesis of (E)-pyrene oxime ester conjugates of carboxylic acids.
- Synthesis of tertiary alcohols based on 1-acetylpyrene.
- Synthesis of (E)-N-[1-(pyren-1-yl)ethylidene]chrysene-2-amine.
- Synthesis of 3,3-di(methylsulfanyl)-1-(1-pyrenyl)-2-propen-1-one.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
391425-10G:
391425-VAR:
391425-BULK:
391425-1G:
この製品を見ている人はこちらもチェック
H Surya Prakash Rao et al.
Beilstein journal of organic chemistry, 3, 31-31 (2007-10-02)
The cycloaddition of the von Leusen's reagent (p-tolylsulfonyl)methyl isocyanide (TosMIC) to alpha-aroylketene dithioacetals (AKDTAs) in the presence of sodium hydride in THF at rt resulted in a facile synthesis of the 4-aroyl-3-methylsulfanyl-2-tosylpyrroles 3 in good yield along with a minor
Synthesis and study of film-forming properties and light sensitivity of 4-acyloxy-3-methoxy (ethoxy) phenylmethylidene-(chrysen-2-yl) amines.
Dikusar EA, et al.
Russ. J. Gen. Chem., 77(2), 278-281 (2007)
Nilanjana Chowdhury et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 11(7), 1239-1250 (2012-05-09)
A new series of (E)-pyrene oxime ester conjugates of carboxylic acids including amino acids were synthesized by coupling with an environment sensitive fluorophore 1-acetylpyrene. (E)-Pyrene oxime esters exhibited strong fluorescence properties and interestingly their fluorescence properties were found to be
Fundamental photoluminescence properties of pyrene carbonyl compounds through absolute fluorescence quantum yield measurement and density functional theory.
Niko Y, et al.
Tetrahedron, 68(31), 6177-6185 (2012)
Synthesis, characterization and pharmacological investigations of some novel heterocyclic derivatives incorporating pyrene and sugar moieties.
Khalifa NM, et al.
Research on Chemical Intermediates, 40(4), 1565-1574 (2014)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)