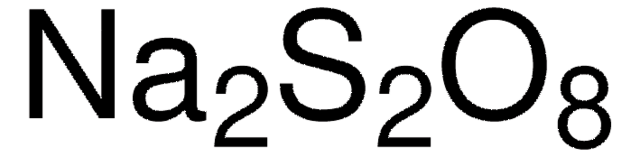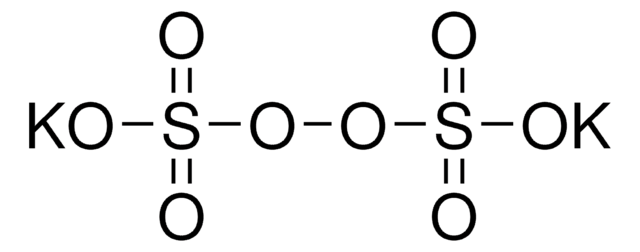S6172
Sodium persulfate
BioXtra, ≥99%
Synonym(s):
Sodium peroxodisulfate
About This Item
Recommended Products
grade
for analytical purposes
Quality Level
product line
BioXtra
Assay
≥99%
reaction suitability
reagent type: oxidant
impurities
<0.0005% Phosphorus (P)
<0.1% Insoluble matter
solubility
H2O: 1 M at 20 °C, clear, colorless
anion traces
chloride (Cl-): <0.05%
cation traces
Al: <0.0005%
Ca: <0.005%
Cu: <0.0005%
Fe: <0.0005%
K: <0.02%
Mg: <0.001%
Pb: <0.001%
Zn: <0.0005%
SMILES string
[Na+].[Na+].[O-]S(=O)(=O)OOS([O-])(=O)=O
InChI
1S/2Na.H2O8S2/c;;1-9(2,3)7-8-10(4,5)6/h;;(H,1,2,3)(H,4,5,6)/q2*+1;/p-2
InChI key
CHQMHPLRPQMAMX-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Application
<li><strong>Electrochemical treatment of organic pollutants in landfill leachate using a three-dimensional electrode system.</strong>: This study explores the electrochemical treatment of landfill leachate using a three-dimensional electrode system. Sodium persulfate is used as an oxidizing agent to degrade organic pollutants effectively, providing a potential method for waste management and environmental protection (Yu et al., 2020).</li>
<li><strong>The Box-Benkhen experimental design for the optimization of the electrocatalytic treatment of wastewaters with high concentrations of phenol and organic matter.</strong>: This paper discusses the optimization of electrocatalytic treatment processes for wastewater containing high levels of phenol and organic matter using sodium persulfate. The study provides valuable insights for improving wastewater treatment efficiency (GilPavas et al., 2009).</li>
<li><strong>Reaction of pectin and glycidyl methacrylate and ulterior formation of free films by reticulation.</strong>: This research involves the chemical modification of pectin with glycidyl methacrylate followed by cross-linking using sodium persulfate, leading to the formation of free-standing films. These films have potential applications in pharmaceuticals and food packaging (Maior et al., 2008).</li>
</ul>
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Ox. Sol. 3 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PRTR
Class I Designated Chemical Substances
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
S6172-VAR:
S6172-1KG:4548173949338
S6172-BULK:
S6172-250G:4548173949345
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service