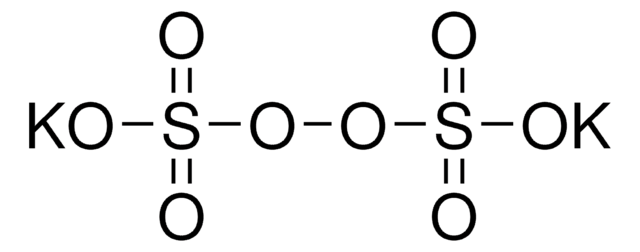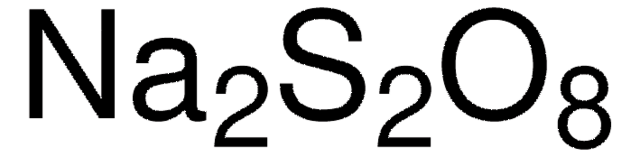60489
Potassium peroxodisulfate
puriss. p.a., ACS reagent, ≥99.0% (RT)
Synonym(s):
Potassium persulfate
About This Item
Recommended Products
grade
ACS reagent
puriss. p.a.
Quality Level
Assay
≥99.0% (RT)
form
powder or crystals
impurities
≤0.001% total nitrogen (N)
pH
2.5-4.5 (25 °C, 27 g/L)
anion traces
chloride (Cl-): ≤10 mg/kg
cation traces
Ag: ≤5 mg/kg
Al: ≤5 mg/kg
Ba: ≤5 mg/kg
Bi: ≤5 mg/kg
Ca: ≤50 mg/kg
Cd: ≤5 mg/kg
Co: ≤5 mg/kg
Cr: ≤5 mg/kg
Cu: ≤5 mg/kg
Fe: ≤5 mg/kg
Li: ≤5 mg/kg
Mg: ≤100 mg/kg
Mn: ≤1 mg/kg
Mo: ≤5 mg/kg
Na: ≤200 mg/kg
Ni: ≤5 mg/kg
Pb: ≤5 mg/kg
Sr: ≤5 mg/kg
Tl: ≤5 mg/kg
Zn: ≤5 mg/kg
application(s)
forensics and toxicology
SMILES string
[K+].[K+].[O-]S(=O)(=O)OOS([O-])(=O)=O
InChI
1S/2K.H2O8S2/c;;1-9(2,3)7-8-10(4,5)6/h;;(H,1,2,3)(H,4,5,6)/q2*+1;/p-2
InChI key
USHAGKDGDHPEEY-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Application
- A polymerization initiator in the synthesis of polyacrylamide via radical polymerization of acrylamide.
- An oxidizing agent in the photochemical oxidation of atrazine aqueous solution.
K2S2O8 can also be used in the following process:
- K2S2O8-AgSCF3 catalytic system is used in the direct trifluoromethylthiolation of unactivated C(sp3)-H bonds under mild conditions. Usage of K2S2O8 in this process plays an important role in the activation of the C-H bond and the oxidation of AgSCF3.
- K2S2O8-FeCl3 is used for the thiocyanation of 2H-indazoles using ammonium thiocyanate as a thiocyanating agent.
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Ox. Sol. 3 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PRTR
Class I Designated Chemical Substances
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
60489-1KG-F:4548173944593
60489-6X1KG-F:
60489-BULK-F:
60489-250G-F:4548173944609
60489-6X250G-F:
60489-VAR-F:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service