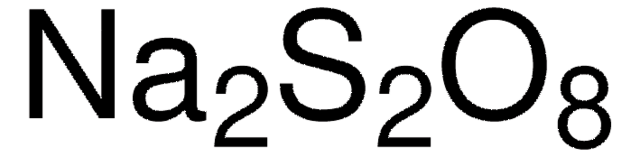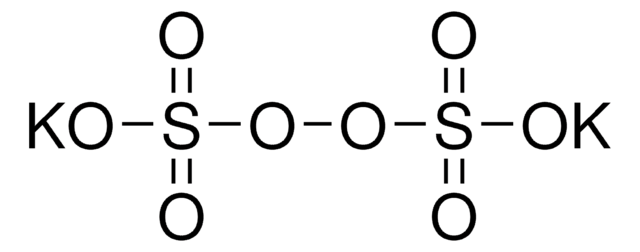216232
Sodium persulfate
reagent grade, ≥98%
Synonym(s):
Sodium peroxodisulfate
About This Item
Recommended Products
grade
reagent grade
Quality Level
Assay
≥98%
form
powder, crystals or granules
reaction suitability
reagent type: oxidant
SMILES string
[Na+].[Na+].[O-]S(=O)(=O)OOS([O-])(=O)=O
InChI
1S/2Na.H2O8S2/c;;1-9(2,3)7-8-10(4,5)6/h;;(H,1,2,3)(H,4,5,6)/q2*+1;/p-2
InChI key
CHQMHPLRPQMAMX-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Application
- For the radical cyclization cascade reaction of 2-alkynylbenzonitriles and sodium arylsulfinates to synthesize sulfonated indenones.
- In the silica-supported aluminum chloride-catalyzed Baeyer-Villiger oxidation of cyclic and acyclic ketones to lactones or esters.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Ox. Sol. 3 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Regulatory Listings
Regulatory Listings are mainly provided for chemical products. Only limited information can be provided here for non-chemical products. No entry means none of the components are listed. It is the user’s obligation to ensure the safe and legal use of the product.
PRTR
Class I Designated Chemical Substances
ISHL Indicated Name
Substances Subject to be Indicated Names
ISHL Notified Names
Substances Subject to be Notified Names
JAN Code
216232-BULK:
216232-500G:4548173931531
216232-2.5KG:4548173931517
216232-1KG:
216232-25G:4548173931524
216232-6X500G:
216232-5KG:
216232-VAR:
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service