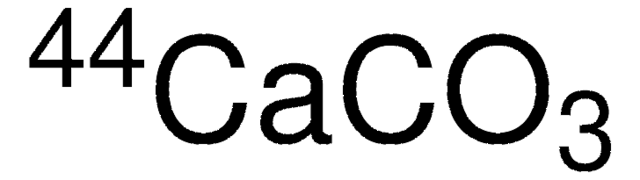LNEHBA1C403
HbA1c in lyophilized hemolysates
LNE Certified Reference Material, commutable, JCTLM listed
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Codice UNSPSC:
41116107
NACRES:
NA.24
Prodotti consigliati
Stato
liquid
Qualità
commutable, JCTLM listed
Durata
limited shelf life, expiry date on the label
Composizione
lyophilized human blood hemolysates, 100%
IVD
not for in vitro diagnostic use
Temperatura di conservazione
2-8°C
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
LNE CRM HbA1c 403 is a higher-order certified reference material produced by the French metrological institute LNE in compliance with ISO 17034 requirements. This Certified Reference Material LNE CRM HbA1c 403 corresponds to lyophilized human blood hemolysates and is intended for use as quality control materials for the verification of the accuracy of the reference method and routine methods used in clinical laboratories for the quantification of HbA1c in hemolysate or whole blood. Sample commutability was assessed through an extensive study involving seventeen of the most used method for the quantification of HbA1c in clinical laboratory using the difference in bias approach described in the IFCC recommendations on commutability assessment. Commutability results are reported in the certificate of analysis.
Cert_ LNE CRM HbA1c 403
Cert_ LNE CRM HbA1c 403
Applicazioni
HbA1c in lyophilized hemolysates (LNE CRM HbA1c 403) is intended for use as quality control material to assess the measurement bias or measurement uncertainty of established or new measurement procedures for the determination of HbA1c in hemolysate or whole blood.
Caratteristiche e vantaggi
- This certified reference material (CRM) ensures accurate HbA1c measurement in clinical diagnostic assays.
- The HbA1c concentration of this material is certified using the IFCC reference method via LC/MS and is derived from real patient samples.
- This CRM is pooled, tested, and certified by the LNE, which includes extensive commutability studies.
- Commutability is assessed for the main routine assays.
- Target values are certified using higher-order reference methods that are calibrated using primary standards of certified purity or certified reference materials, which allows the establishment of metrological traceability of results to the SI units.
- The accuracy of the reference methods used to certify target values is verified using certified matrix reference materials.
- Certified reference materials referenced by the JCTLM as higher order standards.
Altre note
- Details on storage, safety, usage, and operating instructions are provided in the comprehensive Certificate of Analysis.
- This clinical matrix CRM is for research and clinical testing applications.
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Lot/Batch Number
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.