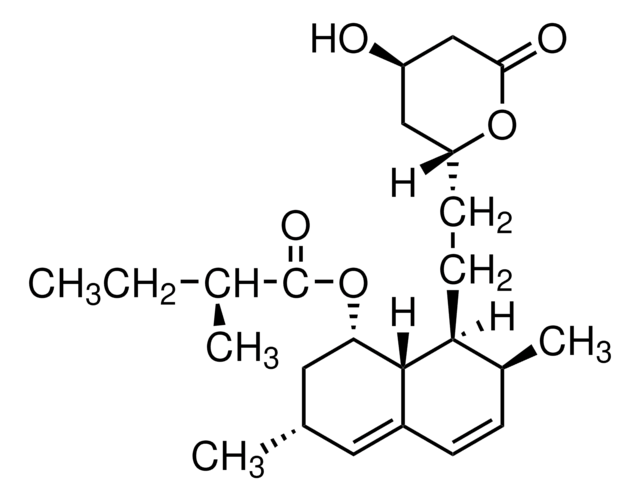
S4194
SR 12813
≥98%, solid
Sinonimo/i:
Tetraethyl 2-(3,5-di-tert-butyl-4-hydroxyphenyl)ethenyl-1,1-bisphosphonate
About This Item
Prodotti consigliati
Origine biologica
synthetic (organic)
Livello qualitativo
Saggio
≥98%
Stato
solid
Solubilità
DMSO: ≥10 mg/mL
H2O: insoluble
Ideatore
GlaxoSmithKline
Stringa SMILE
CCOP(=O)(OCC)C(=C/c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C)\P(=O)(OCC)OCC
InChI
1S/C24H42O7P2/c1-11-28-32(26,29-12-2)21(33(27,30-13-3)31-14-4)17-18-15-19(23(5,6)7)22(25)20(16-18)24(8,9)10/h15-17,25H,11-14H2,1-10H3
YQLJDECYQDRSBI-UHFFFAOYSA-N
Applicazioni
Azioni biochim/fisiol
Caratteristiche e vantaggi
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
The amount of cholesterol that is synthesized in the liver is tightly regulated by dietary cholesterol levels. LDL receptors regulate the cellular transport of lipid rich low density lipoprotein (LDL) particles.
Randomized controlled clinical studies have suggested 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are effective in both primary and secondary prevention of cardiovascular disease (CVD) events.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.