I7634
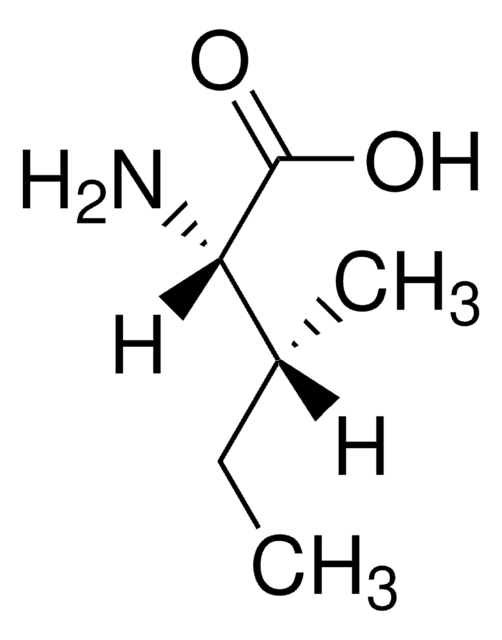
D-Isoleucine
≥98% (TLC)
Sinonimo/i:
(2R, 3R)-2-Amino-3-methylpentanoic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C2H5CH(CH3)CH(NH2)CO2H
Numero CAS:
Peso molecolare:
131.17
Numero CE:
Numero MDL:
Codice UNSPSC:
12352209
ID PubChem:
NACRES:
NA.26
Prodotti consigliati
Nome del prodotto
D-Isoleucine, ≥98% (TLC)
Livello qualitativo
Saggio
≥98% (TLC)
Stato
powder
Impurezze
≤10% allo-isomer
Colore
white
applicazioni
cell analysis
Stringa SMILE
CC[C@@H](C)[C@@H](N)C(O)=O
InChI
1S/C6H13NO2/c1-3-4(2)5(7)6(8)9/h4-5H,3,7H2,1-2H3,(H,8,9)/t4-,5-/m1/s1
AGPKZVBTJJNPAG-RFZPGFLSSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- Metabolic Profiling of Urinary Chiral Amino-Containing Biomarkers for Gastric Cancer Using a Sensitive Chiral Chlorine-Labeled Probe by HPLC-MS/MS.: This study by Huang et al. (2021) explores the use of D-Isoleucine as a biomarker for gastric cancer, utilizing advanced metabolic profiling techniques with HPLC-MS/MS to detect chiral amino-containing compounds in urine samples (Huang et al., 2021).
- Isopenicillin N Synthase Binds δ-(L-α-aminoadipoyl)-L-cysteinyl-D-thia-allo-isoleucine Through Both Sulfur Atoms.: Clifton et al. (2011) describe the binding mechanism of Isopenicillin N synthase with a compound involving D-Isoleucine, highlighting its role in antibiotic biosynthesis and enzyme interaction studies (Clifton et al., 2011).
- The Total Structure of the Antibiotic Longicatenamycin.: Shiba and Mukunoki (1975) determined the complete structure of the antibiotic longicatenamycin, which includes D-Isoleucine as a crucial component, contributing to understanding its antibacterial properties (Shiba and Mukunoki, 1975).
Azioni biochim/fisiol
D-Isoleucine may be used to help characterize and differentiate various D-amino acid oxidases. D-Isoleucine may be used to differentiate the activities of D- and L-isoleucine in processes such as the induction of pigmentation in B16F0 melanoma cells.
Applicazioni
N° Catalogo
Descrizione
Determinazione del prezzo
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Shouji Takahashi et al.
Journal of biochemistry, 159(3), 371-378 (2015-11-01)
D-Aspartate oxidase (DDO) catalyzes the oxidative deamination of acidic D-amino acids, whereas neutral and basic D-amino acids are substrates of D-amino acid oxidase (DAO). DDO of the yeast Cryptococcus humicola (ChDDO) has much higher substrate specificity to D-aspartate, but the
Masago Ishikawa et al.
Biological & pharmaceutical bulletin, 30(4), 677-681 (2007-04-06)
Amino acids, the building blocks of proteins, play significant roles in numerous physiological events in mammals. As the effects of amino acids on melanogenesis have yet to be demonstrated, the present study was conducted to identify whether amino acids, in
Molecular aggregation in selected crystalline 1:1 complexes of hydrophobic D- and L-amino acids. IV. The L-phenylalanine series.
Gorbitz CH, Rissanen K, Valkonen A, Husab? A.
Acta Crystallographica Section C, Crystal Structure Communications, 65, 267-272 (2009)
A Gholizadeh et al.
Biochemistry. Biokhimiia, 74(2), 137-144 (2009-03-10)
D-Amino acid oxidase (DAAO) is an FAD-dependent enzyme that metabolizes D-amino acids in microbes and animals. However, such ability has not been identified in plants so far. We predicted a complete DAAO coding sequence consisting of 1158 bp and encoding
Ivan Kashkan et al.
Plants (Basel, Switzerland), 9(7) (2020-07-12)
Rapid progress in plant molecular biology in recent years has uncovered the main players in hormonal pathways and characterized transcriptomic networks associated with hormonal response. However, the role of RNA processing, in particular alternative splicing (AS), remains largely unexplored. Here
Chromatograms
application for HPLCIl team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.