C7757
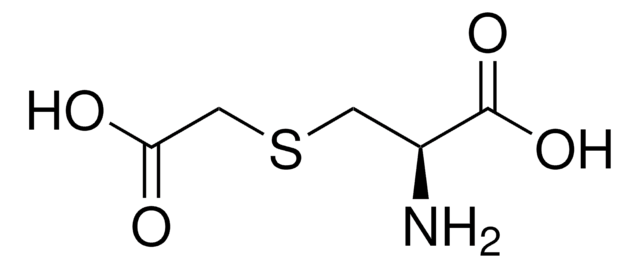
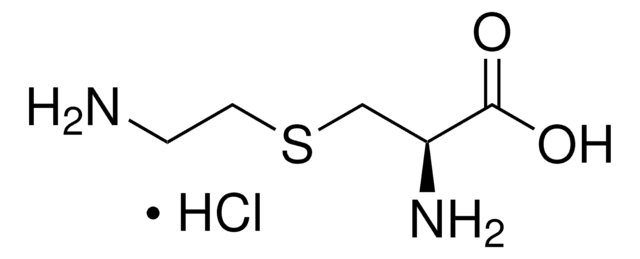
S-Carboxymethyl-L-cysteine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
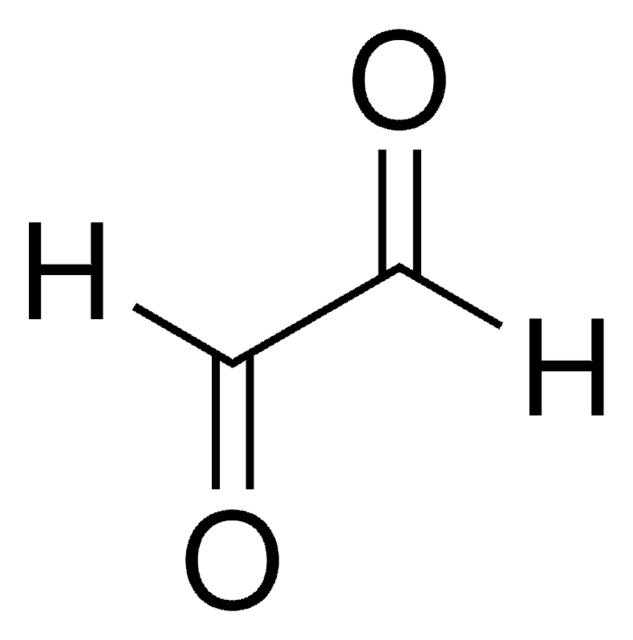
C5H9NO4S
Numero CAS:
Peso molecolare:
179.19
Beilstein:
1725012
Numero CE:
Numero MDL:
Codice UNSPSC:
12352209
ID PubChem:
NACRES:
NA.26
Prodotti consigliati
Saggio
>98% (TLC)
Stato
powder
tecniche
cell culture | mammalian: suitable
Colore
white
Punto di fusione
200 °C
Temperatura di conservazione
2-8°C
Stringa SMILE
N[C@@H](CSCC(O)=O)C(O)=O
InChI
1S/C5H9NO4S/c6-3(5(9)10)1-11-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1
GBFLZEXEOZUWRN-VKHMYHEASA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Azioni biochim/fisiol
S-Carboxymethyl-L-cysteine is studied as a small molecule mucoactive drug in vivo. These studies include analyzing the oxidative metabolism of S-carboxymethyl-L-cysteine by enzymes such as phenylalanine monooxygenase(s).
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Recombinant heteromeric phenylalanine monooxygenase and the oxygenation of carbon and sulfur substrates.
Boonyapiwat B, Mitchell SC, et al.
J. Pharm. Pharm. Sci., 63, 558-564 (2011)
Human phenylalanine monooxygenase and thioether metabolism.
Boonyapiwat B, Panaretou B, et al.
J. Pharm. Pharm. Sci., 61, 63-67 (2009)
Marty K Soehnlen et al.
The Journal of antimicrobial chemotherapy, 66(3), 574-577 (2011-03-12)
To screen novel small molecule compounds for inhibition of Mycoplasma bovis growth and to characterize their activity in terms of dose-dependency and ability to function in milk. Using a tetrazolium salt cytotoxicity assay, 480 natural compounds were screened to determine
Panayotis Panagopoulos et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 39(4), 219-223 (2009-12-29)
The aim of this study was to investigate the feasibility of employing S-carboxymethyl-L-cysteine as a treatment of chronic obstructive pulmonary disease in dogs. To this end the pharmacokinetic parameters of orally administered S-carboxymethyl-L-cysteine were determined in the dog, cow and
Masanori Asada et al.
Respiratory physiology & neurobiology, 180(1), 112-118 (2011-11-15)
To examine the effects of l-carbocisteine on airway infection with respiratory syncytial (RS) virus, human tracheal epithelial cells were pretreated with l-carbocisteine and infected with RS virus. Viral titer, virus RNA, and pro-inflammatory cytokine secretion, including interleukin (IL)-1 and IL-6
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.