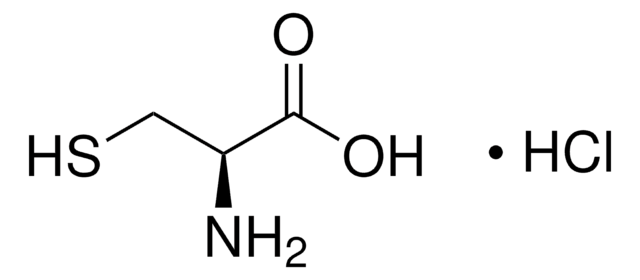
A2636
S-(2-Aminoethyl)-L-cysteine hydrochloride
≥98% (TLC)
Sinonimo/i:
L-4-Thialysine hydrochloride
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C5H12N2O2S · HCl
Numero CAS:
Peso molecolare:
200.69
Beilstein:
3697262
Numero CE:
Numero MDL:
Codice UNSPSC:
12352209
eCl@ss:
32160406
ID PubChem:
NACRES:
NA.26
Prodotti consigliati
Nome del prodotto
S-(2-Aminoethyl)-L-cysteine hydrochloride, ≥98% (TLC)
Livello qualitativo
Saggio
≥98% (TLC)
Stato
powder
Colore
white to off-white
Temperatura di conservazione
2-8°C
Stringa SMILE
Cl.NCCSC[C@H](N)C(O)=O
InChI
1S/C5H12N2O2S.ClH/c6-1-2-10-3-4(7)5(8)9;/h4H,1-3,6-7H2,(H,8,9);1H/t4-;/m0./s1
CVHKULVNPGAEQM-WCCKRBBISA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Azioni biochim/fisiol
S-(2-Aminoethyl)-L-cysteine (AEC) hydrochloride is used as a lysine analogue for comparative analysis with other lysine analogues. S-(2-Aminoethyl)-L-cysteine is an alternative substrate useful for characterizing lysine cyclodeaminase from Streptomyces pristinaespiralis. AEC may be used as a non-antibiotic selection agent for genetically engineered soybeans expressing a lysine insensitive DHPS gene. AEC is being studied as an amino acid antibiotic.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Asri Peni Wulandari et al.
FEBS letters, 522(1-3), 35-40 (2002-07-04)
In Thermus thermophilus homocitrate synthase (HCS) catalyzes the initial reaction of lysine biosynthesis through alpha-aminoadipic acid, synthesis of homocitrate from 2-oxoglutarate and acetyl-CoA. HCS is strongly inhibited by lysine, indicating that the biosynthesis is regulated by the endproduct at the
M N Cahyanto et al.
Journal of applied microbiology, 102(3), 674-679 (2007-02-21)
To enhance L-lysine secretion in Lactobacillus plantarum. An S-2-aminoethyl-L-cystein (AEC)-resistant mutant of L. plantarum was isolated, and it produced L-lysine at considerably higher level than the parent strain. Aspartokinase in the mutant has been desensitized to feedback inhibition by L-lysine.
Dasantila Golemi-Kotra et al.
The Journal of biological chemistry, 279(33), 34665-34673 (2004-05-21)
Beta-lactamases and penicillin-binding proteins are bacterial enzymes involved in antibiotic resistance to beta-lactam antibiotics and biosynthetic assembly of cell wall, respectively. Members of these large families of enzymes all experience acylation by their respective substrates at an active site serine
Amarendra N Maity et al.
The journal of physical chemistry. B, 113(36), 12161-12163 (2009-08-19)
We demonstrate that the steady state reaction of lysine 5,6-aminomutase with substrate analogue 4-thia-l-lysine generates a radical intermediate, which accumulates in the enzyme to an electron paramagnetic resonance (EPR) detectable level. EPR line width narrowing of approximately 1 mT due
Do Youn Jun et al.
Biochemical pharmacology, 66(12), 2291-2300 (2003-11-26)
We first report the mechanism for the inhibitory effect of the lysine analog, thialysine on human acute leukemia Jurkat T cells. When Jurkat T cells were treated with thialysine (0.32-2.5 mM), apoptotic cell death along with several biochemical events such
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.