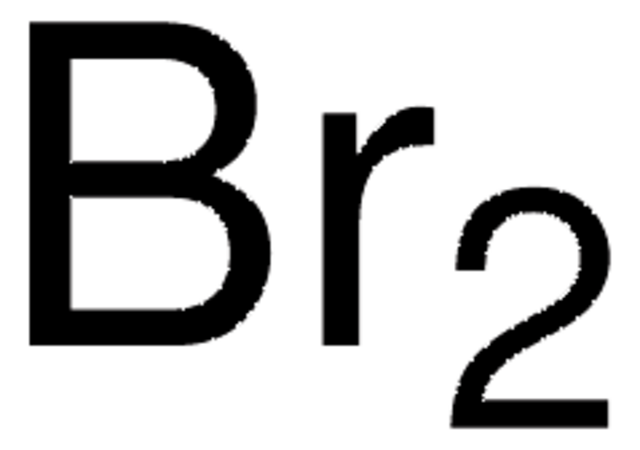277576
Bromina
ACS reagent, ≥99.5%
About This Item
Prodotti consigliati
Grado
ACS reagent
Livello qualitativo
Densità del vapore
7.14 (vs air)
Tensione di vapore
175 mmHg ( 20 °C)
671 mmHg ( 55 °C)
Saggio
≥99.5%
Stato
liquid
Resistività
7.8E18 μΩ-cm, 20°C
Impurezze
organic bromine compounds, passes test
≤0.001% I2
≤0.001% S compounds
Residuo dopo evaporazione
≤0.005%
P. ebollizione
58.8 °C (lit.)
Punto di fusione
−7.2 °C (lit.)
Densità
3.119 g/mL at 25 °C (lit.)
Anioni in tracce
chloride (Cl-): ≤0.05%
Cationi in tracce
Ni: ≤5 ppm
heavy metals: ≤2 ppm
Stringa SMILE
BrBr
InChI
1S/Br2/c1-2
GDTBXPJZTBHREO-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Aromatic and heterocyclic compounds.
- Carbonyl compounds at α position to carbonyl groups.
- Aromatic carboxylic acids via Hell-Volhard-Zelinski reaction in the presence of phosphorus trihalides.
- Alkylbenzenes at the benzylic position using photocatalytic conditions.
It can also be used as an oxidizing agent for the oxidation of:
- Primary alcohols to either aldehydes or esters.
- Secondary alcohols to ketones.
It can also be used as a Lewis acid catalyst to:
- Convert epoxides and CO2 to cyclic carbonates in a continuous flow system.
- Synthesize bis(indolyl)methanes by reacting indoles with carbonyl compounds.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 1 Inhalation - Aquatic Acute 1 - Eye Dam. 1 - Skin Corr. 1A
Codice della classe di stoccaggio
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.