78181
Phenylboronic acid
purum, ≥97.0% (HPLC)
Sinonimo/i:
Benzeneboronic acid, Dihydroxyphenylborane, NSC 66487, Phenyl-boric acid, Phenylboric acid, Phenyldihydroxyborane
About This Item
Prodotti consigliati
Grado
purum
Livello qualitativo
Saggio
≥97.0% (HPLC)
Stato
crystals
Punto di fusione
216-219 °C (lit.)
218-222 °C
Stringa SMILE
OB(O)c1ccccc1
InChI
1S/C6H7BO2/c8-7(9)6-4-2-1-3-5-6/h1-5,8-9H
HXITXNWTGFUOAU-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Phenylboronic acid (PBA) is an organoboronic acid. It behaves as a molecular receptor that can attach to compounds containing cis-diol group. Microwave-assisted Suzuki coupling of aryl chlorides with phenylboronic acid in the presence of Pd/C (catalyst) and water (solvent) has been described. Palladium-catalyzed cross-coupling reaction of phenylboronicacid with haloarenes to afford biaryls has been reported.
Applicazioni
- Rhodium-catalyzed intramolecular amination.
- Pd-catalyzed direct arylation.
- Mizoroki-Heck and Suzuki-Miyaura coupling reactions catalyzed by palladium nanoparticles.
- Palladium-catalyzed stereoselective Heck-type reaction.
- Highly effective Palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water.
Phenylboronic acid may be employed as reagent in the preparation of:
- Ni(II) pincer complex and Pd(II) pyridoxal hydrazone metallacycles as catalysts for the Suzuki-Miyaura cross-coupling reactions.
- N-type polymers for all-polymer solar cells.
- Novel series of potent and selective mTOR kinase inhibitors.
- Inhibitors of lactate dehydrogenase against cancer cell proliferation.
Altre note
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
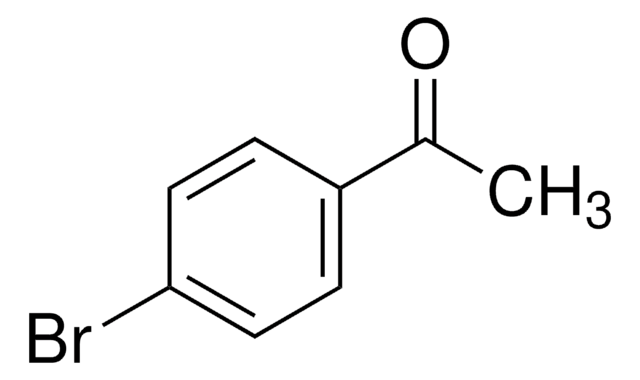
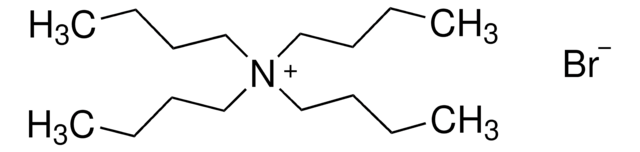
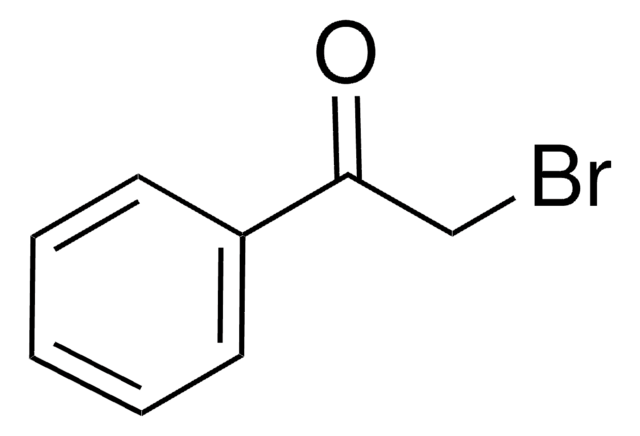
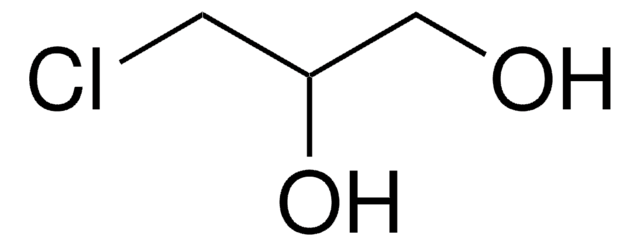
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)