51799
Putrescine
analytical standard
Sinonimo/i:
1,4-Diaminobutane, 1,4-Butanediamine, Putrescine, Tetramethylenediamine
About This Item
Prodotti consigliati
Grado
analytical standard
Livello qualitativo
Saggio
≥98.5% (GC)
Durata
limited shelf life, expiry date on the label
Limite di esplosione
9.08 %
tecniche
HPLC: suitable
gas chromatography (GC): suitable
Indice di rifrazione
n20/D 1.457 (lit.)
P. eboll.
158-160 °C (lit.)
Punto di fusione
25-28 °C (lit.)
Densità
0.877 g/mL at 25 °C (lit.)
applicazioni
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
Formato
neat
Temperatura di conservazione
2-8°C
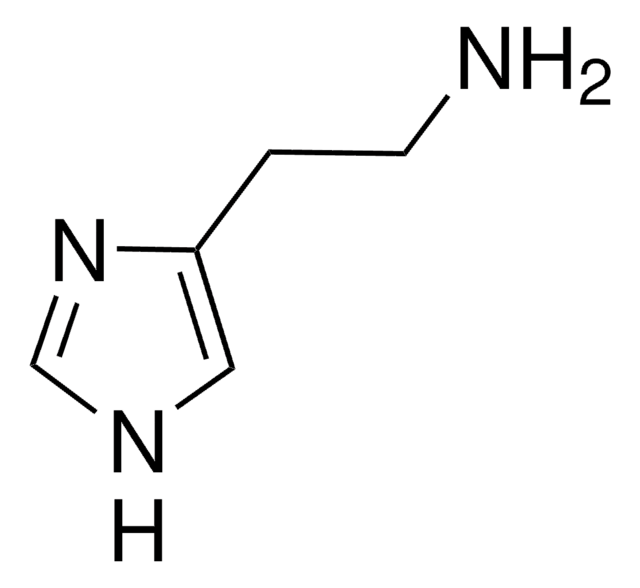
Stringa SMILE
NCCCCN
InChI
1S/C4H12N2/c5-3-1-2-4-6/h1-6H2
KIDHWZJUCRJVML-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Putrescine in food analysis: A study demonstrated a novel method for the selective extraction of dietary polyamines, including putrescine, from chicken breast, utilizing lab-on-a-chip electromembrane and dispersive liquid-liquid microextraction techniques for enhanced food analysis (Barzegar et al., 2024).
- Putrescine in biochemical analysis: The application of Electrostatic Repulsion Hydrophilic Interaction Liquid Chromatography (ERLIC) for the quantitative analysis of polyamines such as putrescine showcases its importance in biochemical assays, providing precise measurement tools for research and development (Dörfel et al., 2024).
Altre note
Prodotti consigliati
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
113.0 °F - closed cup
Punto d’infiammabilità (°C)
45 °C - closed cup
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Protocolli
HPLC Analysis of Biogenic Amines on Ascentis® RP-Amide
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.