85590
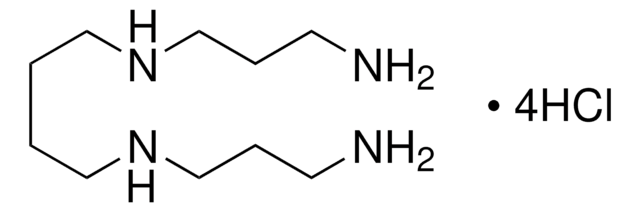
Spermine
≥99.0% (GC)
Sinonimo/i:
N,N′-Bis(3-aminopropyl)-1,4-diaminobutane, Gerontine, Musculamine, Neuridine
About This Item
Prodotti consigliati
Origine biologica
microbial
synthetic
Livello qualitativo
Saggio
≥99.0% (GC)
Forma fisica
crystalline (chunks)
Impurezze
≤0.5% spermidine
P. eboll.
150 °C/5 mmHg (lit.)
Punto di fusione
28-30 (lit.)
Solubilità
water: 0.05 g/mL, clear, colorless
Anioni in tracce
carbonate (CO32-): ≤5000 mg/kg
Temperatura di conservazione
2-8°C
Stringa SMILE
[H]N(CCCN)CCCCN([H])CCCN
InChI
1S/C10H26N4/c11-5-3-9-13-7-1-2-8-14-10-4-6-12/h13-14H,1-12H2
PFNFFQXMRSDOHW-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
Azioni biochim/fisiol
Prodotti correlati
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Eye Dam. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Mass Spectrometry of Glycans, method comparison and products
Protocolli
HPLC Analysis of Biogenic Amines on Ascentis® RP-Amide
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.