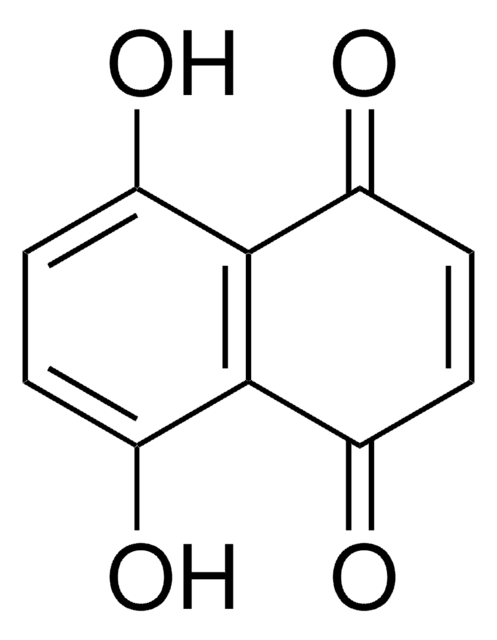
H47003
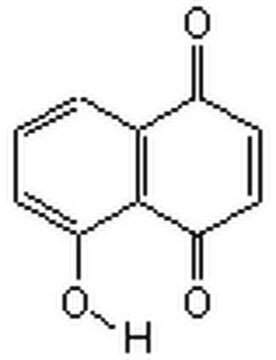
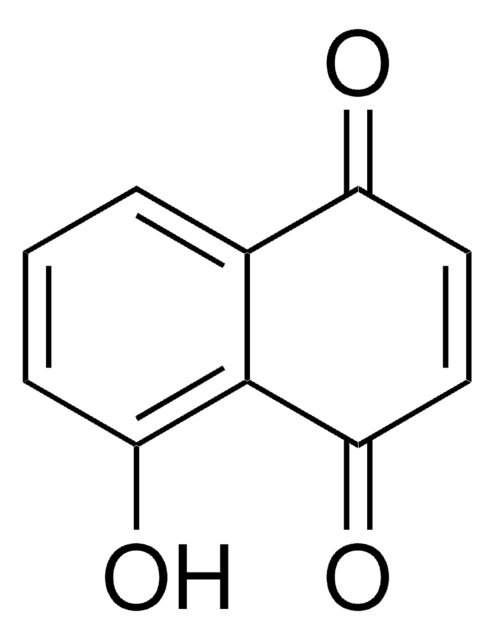
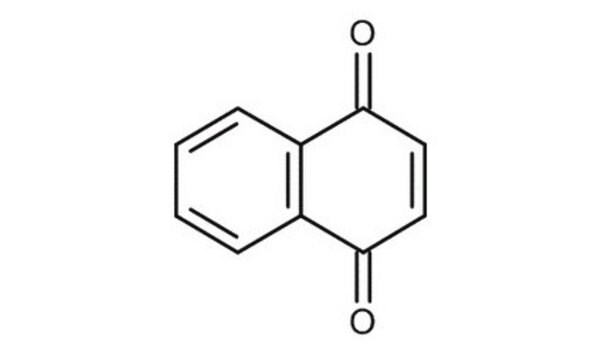
5-Hydroxy-1,4-naphthoquinone
97%
Sinonimo/i:
Juglone
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H6O3
Numero CAS:
Peso molecolare:
174.15
Beilstein:
1909764
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Punto di fusione
161-163 °C (lit.)
Stringa SMILE
Oc1cccc2C(=O)C=CC(=O)c12
InChI
1S/C10H6O3/c11-7-4-5-9(13)10-6(7)2-1-3-8(10)12/h1-5,12H
KQPYUDDGWXQXHS-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
5-Hydroxy-1,4-naphthoquinone (juglone) can be used as a starting material for the synthesis of:
Juglone can also be used as a dienophile in the Diels–Alder reaction for the synthesis of variety of C-aryl glycosides.
- Trypanocidal drugs.
- Tacrine-naphthoquinone hybrids with potential application in the treatment of Alzheimer′s disease.
- Juglone-based electroactive polymer, poly(5-hydroxy-1,4-naphthoquinone-co-5-hydroxy-3-thioacetic acid-1,4-naphthoquinone), for the electrochemical detection of DNA hybridization.
- Ent-nocardione A, naturally-occurring tyrosine phosphatase inhibitor.
Juglone can also be used as a dienophile in the Diels–Alder reaction for the synthesis of variety of C-aryl glycosides.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Multitarget drug design strategy: quinone-tacrine hybrids designed to block amyloid-β aggregation and to exert anticholinesterase and antioxidant effects.
Nepovimova E, et al.
Journal of medicinal chemistry, 57(20), 8576-8589 (2014)
Application of an Enyne Metathesis/Diels-Alder Cycloaddition Sequence: A New Versatile Approach to the Syntheses of C-Aryl Glycosides and Spiro-C-Aryl Glycosides.
Subrahmanyam AV, et al.
Chemistry?A European Journal, 16(28), 8545-8556 (2010)
2-and 3-substituted 1, 4-naphthoquinone derivatives as subversive substrates of trypanothione reductase and lipoamide dehydrogenase from trypanosoma c ruzi: synthesis and correlation between redox cycling activities and in vitro cytotoxicity.
Salmon-Chemin L, et al.
Journal of Medicinal Chemistry, 44(4), 548-565 (2001)
Study of the DNA hybridization transduction behavior of a quinone-containing electroactive polymer by cyclic voltammetry and electrochemical impedance spectroscopy.
Piro B, et al.
Journal of Electroanalytical Chemistry, 577(1), 155-165 (2005)
Formal Radical Cyclization onto Benzene Rings: A General Method and Its Use in the Synthesis of ent-Nocardione A.
Clive DLJ, et al.
The Journal of Organic Chemistry, 69(10), 3282-3293 (2004)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.