722332
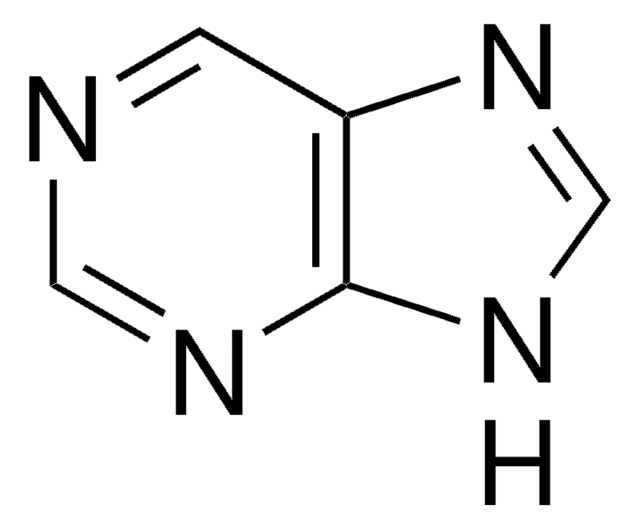
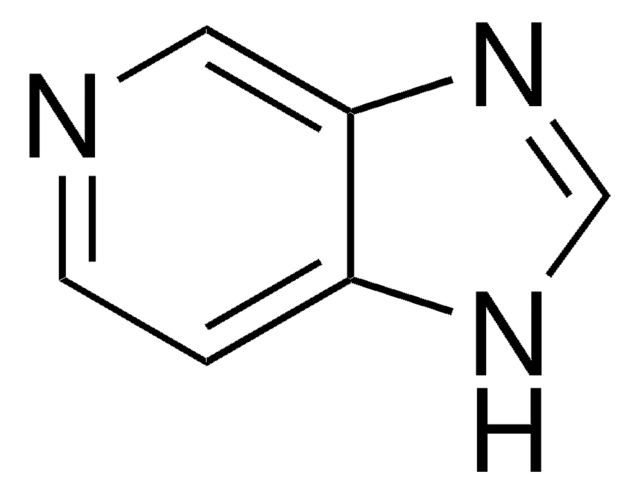
6-Amino-7-deazapurine
97%
Sinonimo/i:
4-Amino-7H-pyrrolo[2,3-d]pyrimidine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H6N4
Numero CAS:
Peso molecolare:
134.14
Numero MDL:
Codice UNSPSC:
12352005
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Stato
solid
Punto di fusione
257-262 °C
Stringa SMILE
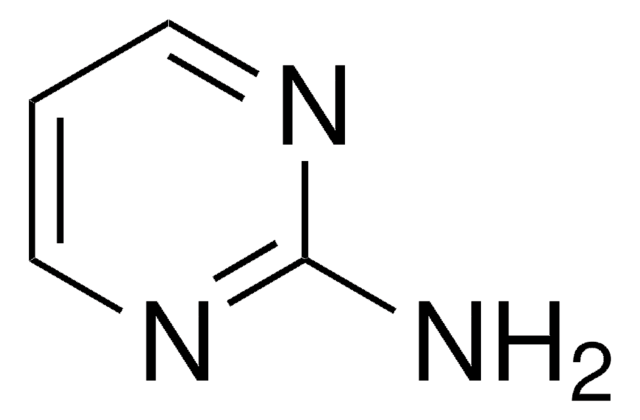
Nc1ncnc2[nH]ccc12
InChI
1S/C6H6N4/c7-5-4-1-2-8-6(4)10-3-9-5/h1-3H,(H3,7,8,9,10)
PEHVGBZKEYRQSX-UHFFFAOYSA-N
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Jennifer D Tibodeau et al.
Inorganic chemistry, 43(2), 408-410 (2004-01-21)
The effect of DNA bending on nucleobase electron transfer was investigated by studying the oxidation of double-stranded sequences containing seven repeats of the known bent sequence d(GGCA(1)A(2)A(3)A(4)A(5)A(6)C) where 7-deazaadenine (zA) was substituted at the A(3) position. Native gel electrophoresis was
Kiyohiko Kawai et al.
Nature chemistry, 1(2), 156-159 (2009-05-01)
Interest in using DNA as a building block for nanoelectronic sensors and devices stems from its efficient hole-conducting properties and the relative ease with which it can be organized into predictable nanometre-sized two- and three-dimensional structures. However, because a hole
A Ono et al.
Nucleic acids research, 12(23), 8939-8949 (1984-12-11)
Deoxydecanucleotides having a recognition sequence of Bgl II and Sau 3AI, and their 7-deazaadenine analogs were synthesized. The decanucleotides containing 7-deazaadenine in place of adenine were partially resistant to the hydrolysis by Sau 3AI and strongly resistant to that by
Pavel Kielkowski et al.
The Journal of organic chemistry, 76(9), 3457-3462 (2011-03-24)
(Cytosin-5-yl)ethynyl derivatives of pyrimidine and 7-deazaadenine 2-deoxyribonucleosides and nucleoside triphosphates (dNTPs) were prepared in one step by the aqueous Sonogashira coupling of unprotected halogenated nucleos(t)ides with 5-ethynylcytosine. The modified dNTPs were good substrates for DNA polymerases suitable for primer extension
Akimitsu Okamoto et al.
Bioorganic & medicinal chemistry letters, 12(1), 97-99 (2001-12-12)
2-Amino-7-deazaadenine ((AD)A) was incorporated into oligodeoxynucleotides (ODN) and their base-pairing properties with natural nucleobases were investigated. In melting temperature (T(m)) experiments, the duplex containing an (AD)A/C base pair showed a high stability comparable to that containing (AD)A/T base pair. Destabilization
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.