261823
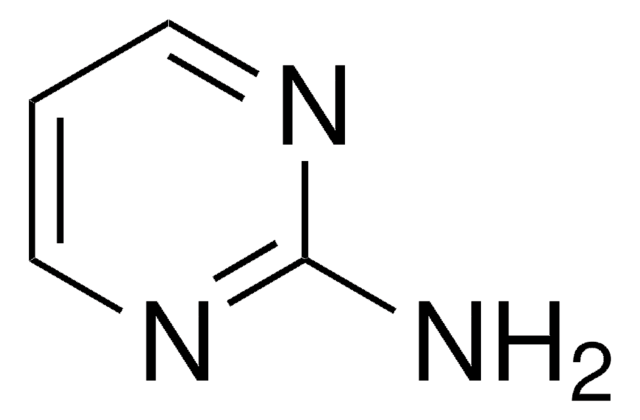
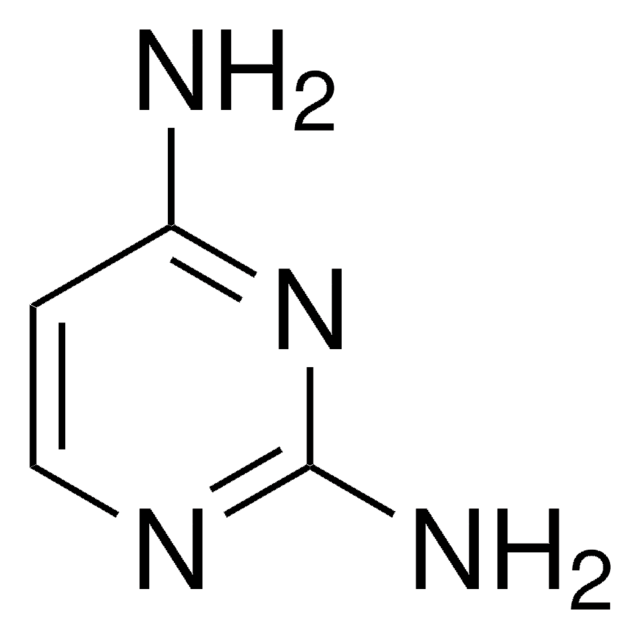
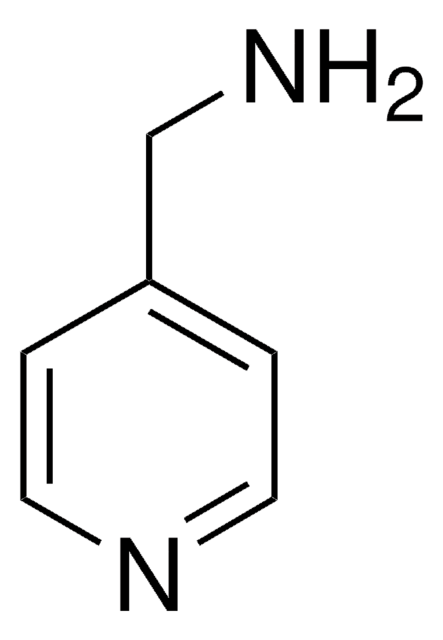
4-Aminopyrimidine
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C4H5N3
Numero CAS:
Peso molecolare:
95.10
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
solid
Punto di fusione
154-156 °C (lit.)
Stringa SMILE
Nc1ccncn1
InChI
1S/C4H5N3/c5-4-1-2-6-3-7-4/h1-3H,(H2,5,6,7)
OYRRZWATULMEPF-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
The consequences of one-electron oxidation and one-electron reduction were studied for 4-aminopyrimidine.
Applicazioni
4-Aminopyrimidine has been used in the preparation of 1:4-dihydro-4-imino-1-methylpyrimidine hydriodide.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Ewa D Raczyńska et al.
Journal of molecular modeling, 18(8), 3523-3533 (2012-02-14)
The consequences of one-electron oxidation and one-electron reduction were studied for 4-aminopyrimidine (4APM), which displays prototropic tautomerism. Since experimental techniques are incapable of detecting less than 0.1% of minor tautomers, quantum-chemical calculations [DFT(B3LYP)/6-311+G(d,p)] were carried out for all possible tautomers
R Friedemann et al.
Biochimica et biophysica acta, 1385(2), 245-250 (1998-07-10)
Ab initio calculations on the HF-SCF 6-31g* level were performed on tautomers as well as protonated and deprotonated species of thiamin. Aspects of the proton relay function of the 4'-aminopyrimidine ring in the thiamin catalysis were studied on model systems.
A Schellenberger
Biochimica et biophysica acta, 1385(2), 177-186 (1998-07-10)
The mechanism of ThDP enzymes originates in the anionic (ylid) structure of the coenzyme. On the other hand, no ylid species (as permanently existing structure) could be detected by 13C2-NMR studies with PDC (yeast), when the cofactor binds to the
Dana Nachtigallová et al.
Journal of the American Chemical Society, 132(24), 8261-8263 (2010-06-02)
Nonadiabatic photodynamical simulations of 4-aminopyrimidine (4-APy) used as a model for adenine were performed by embedding it between two stacking methyl-guanine (mGua) molecules to determine the effect of spatial restrictions on the ultrafast photodeactivation mechanism of this nucleobase. A hybrid
Potential roles of the aminopyrimidine ring in thiamin catalyzed reactions.
F Jordan et al.
Annals of the New York Academy of Sciences, 378, 14-31 (1982-01-01)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.