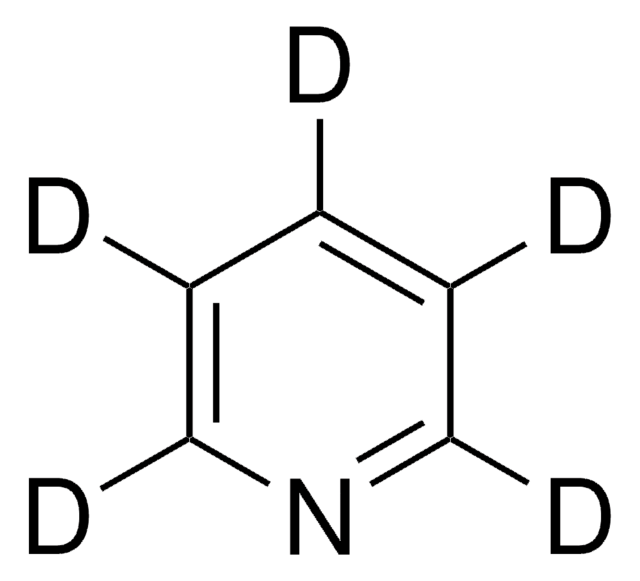
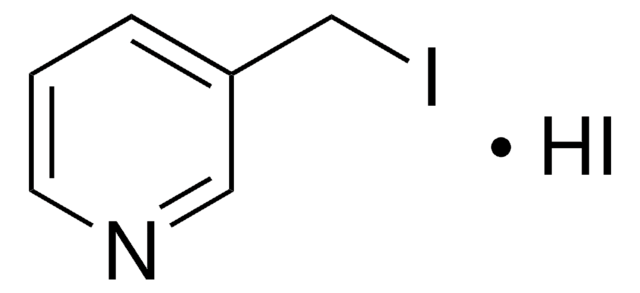
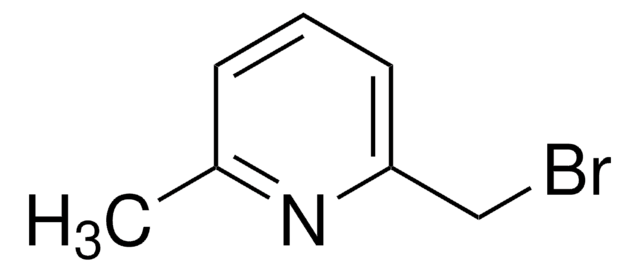
491748
4-(Bromomethyl)pyridine hydrobromide
97%
Sinonimo/i:
(4-Pyridyl)methyl bromide hydrobromide, 4-(Bromomethyl)pyridine monohydrobromide, 4-Picolyl bromide hydrobromide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H6BrN · HBr
Numero CAS:
Peso molecolare:
252.93
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
97%
Punto di fusione
189-192 °C (lit.)
Stringa SMILE
Br[H].BrCc1ccncc1
InChI
1S/C6H6BrN.BrH/c7-5-6-1-3-8-4-2-6;/h1-4H,5H2;1H
VAJUUDUWDNCECT-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
4-(Bromomethyl)pyridine hydrobromide is a substituted pyridine. It reacts with 1,2-ethanediamine and 1,3-propanediamine to form the corresponding diamines.
Applicazioni
4-(Bromomethyl)pyridine hydrobromide may be used in the preparation of:
- 3-(4-pyridylmethyl)-2′,3′-di-O-oleyl-5′-O-(4,4′-dimethoxytriphenylmethyl)uridine
- 3-(4-pyridylmethyl)-3′-O-oleyl-5′-O-(4,4-dimethoxytriphenylmethyl)-thymidine
- 1,4-bis(N-hexyl-4-pyridinium)butadiene diperchlorate
- 2-morpholin-4-yl-7-(pyridin-4-ylmethoxy)-4H-1,3-benzoxazin-4-one
- 8-methyl-2-morpholin-4-yl-7-(pyridin-4-ylmethoxy)-4H-1,3-benzoxazin-4-one
- 2-morpholin-4-yl-8-(pyridin-4-ylmethoxy)-4H-1,3-benzoxazin-4-one
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Luca Simeone et al.
Molecular bioSystems, 7(11), 3075-3086 (2011-09-08)
Novel thymidine- or uridine-based nucleolipids, containing one hydrophilic oligo(ethylene glycol) chain and one or two oleic acid residues (called ToThy, HoThy and DoHu), have been synthesized with the aim to develop bio-compatible nanocarriers for drug delivery and/or produce pro-drugs. Microstructural
Photoinduced electron transfer in supramolecular complexes of a p-extended viologen with porphyrin monomer and dimer.
Fukuzumi S, et al.
Royal Society of Chemistry Advances, 2(9), 3741-3747 (2012)
Cristiane F da Costa et al.
Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 63(1), 40-42 (2008-02-12)
We report in this work the preparation and the in vitro antileishmanial activity of a series of long chains N-monoalkylated diamines and two pyridinediamine derivatives. Several compounds, tested for their in vitro antiproliferative activity against Leishmania amazonensis and Leishmania chagasi
Saleh Ihmaid et al.
European journal of medicinal chemistry, 45(11), 4934-4946 (2010-08-31)
A number of new 2-amino-[5, 6, 7 and 8]-O-substituted 1,3-benzoxazines, and 2-amino 8-methyl-7-O-substituted-1,3-benzoxazines were synthesized. Thirty one new compounds were tested for their effect on collagen induced platelet aggregation and it was found that the most active compounds were 8-methyl-2-morpholin-4-yl-7-(pyridin-3-ylmethoxy)-4H-1,3-benzoxazin-4-one
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.