475262
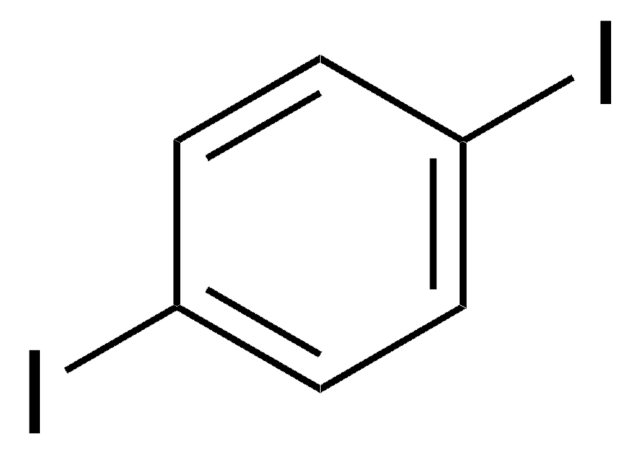
1,3-Diiodobenzene
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H4I2
Numero CAS:
Peso molecolare:
329.90
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Condizioni di stoccaggio
protect from light
Punto di fusione
34-37 °C (lit.)
Gruppo funzionale
iodo
Temperatura di conservazione
2-8°C
Stringa SMILE
Ic1cccc(I)c1
InChI
1S/C6H4I2/c7-5-2-1-3-6(8)4-5/h1-4H
SFPQFQUXAJOWNF-UHFFFAOYSA-N
Descrizione generale
1,3-Diiodobenzene is a halogenated benzene derivative. Its reaction with phenylboronic acid in the presence of CuI, DABCO (1,4-diazabicyclo[2.2.2]octane) and TBAB (n-Bu4NBr) has been analyzed. 1,3-Diiodobenzene undergoes coupling with 2-methylthiophene in the presence of Ir/Ag2CO3 to afford meta-linked isomer of thiophene-benzene-thiophene triad.
Applicazioni
1,3-Diiodobenzene may be used in the synthesis of:
- 3,5-bis(perfluorodecyl)phenylboronic acid
- epitaxially aligned and separated polyphenylene lines on Cu(110)
- 1,3-bis(4-ethynyl-2,5-dibutoxyphenyl-1-ethynyl)benzene
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
235.4 °F - closed cup
Punto d’infiammabilità (°C)
113 °C - closed cup
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Jin-Heng Li et al.
The Journal of organic chemistry, 72(6), 2053-2057 (2007-02-09)
In the presence of TBAB, CuI-catalyzed Suzuki-Miyaura cross-coupling of vinyl halides and aryl halides with arylboronic acids was conducted smoothly to afford the corresponding diarylethenes and polyaryls in moderate to good yields using DABCO (1,4-diazabicyclo[2.2.2]octane) as the ligand. We also
Benoît Join et al.
Angewandte Chemie (International ed. in English), 48(20), 3644-3647 (2009-04-09)
Efficient couplings using equimolar quantities of each coupling partner and multiple C-H bond arylation reactions are achieved with an Ir-based catalytic system for the C-H bond arylation of electron-rich heteroarenes with iodoarenes to construct extended pi-systems. The dramatic ligand effect
J A Lipton-Duffin et al.
Small (Weinheim an der Bergstrasse, Germany), 5(5), 592-597 (2009-02-26)
The surface-mediated synthesis of epitaxially aligned and separated polyphenylene lines on Cu(110) by exploiting the Ullmann dehalogenation reaction is reported. Scanning tunneling microscopy (STM) and X-ray photoelectron spectroscopy (XPS) show that the C-I bonds of 1,4-diiodobenzene and 1,3-diiodobenzene (C(6)H(4)I(2)) are
Synthesis, Chain Rigidity, and Luminescent Properties of Poly [(1, 3-phenyleneethynylene)-a lt-tris (2, 5-dialkoxy-1, 4-phenyleneethynylene)] s.
Chu Q, et al.
Macromolecules, 35(20), 7569-7574 (2002)
3, 5-Bis (perfluorodecyl) phenylboronic acid as an easily recyclable direct amide condensation catalyst.
Ishihara K, et al.
Synlett, 2001(09), 1371-1374 (2001)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.