171565
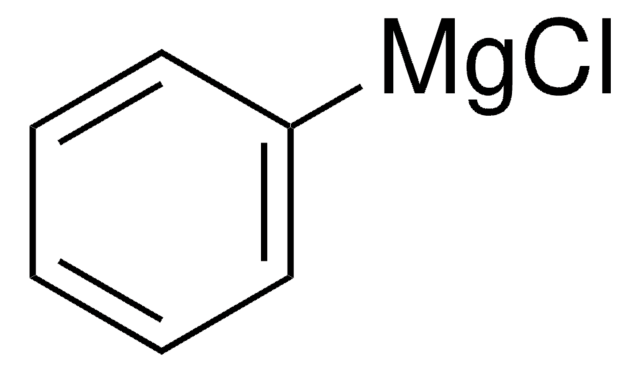
Phenylmagnesium bromide solution
3.0 M in diethyl ether
Sinonimo/i:
Bromomagnesiobenzene, Bromophenylmagnesium
About This Item
Prodotti consigliati
Livello qualitativo
Impiego in reazioni chimiche
reaction type: Grignard Reaction
Concentrazione
3.0 M in diethyl ether
Densità
1.134 g/mL at 25 °C
Stringa SMILE
Br[Mg]c1ccccc1
InChI
1S/C6H5.BrH.Mg/c1-2-4-6-5-3-1;;/h1-5H;1H;/q;;+1/p-1
ANRQGKOBLBYXFM-UHFFFAOYSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
It may be used for synthesis of the following:
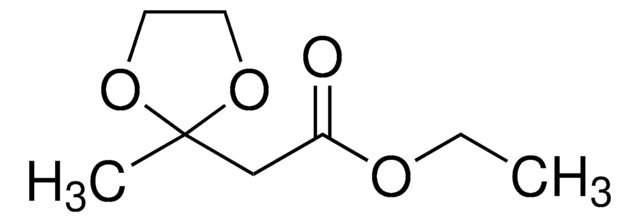
- 1,3,3-trimethyl-6-phenyl-2-oxabicyclo[2.2.2]octan-6-ol
- 6-benzyl-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-ol
- (3-(2-Dithiobenzoatepropionyl)propyl)dimethylmethoxysilane, reversible addition-fragmentation chain transfer polymerization (RAFT)-silane agent
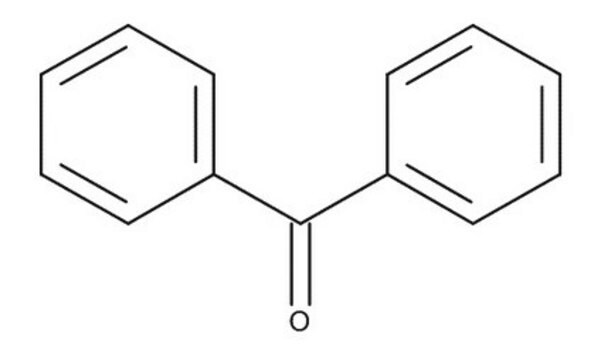
- series of o-substituted benzophenones
Confezionamento
Altre note
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
Organi bersaglio
Respiratory system
Rischi supp
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
-40.0 °F - closed cup
Punto d’infiammabilità (°C)
-40 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| S892858-1EA | |
| 171565-18L | 4061838751065 |
| 171565-20L | |
| 171565-50ML | 4061838751072 |
| 171565-800ML | 4061838751089 |
| 171565-100ML | 4061838751058 |
| 171565-18L-C | 4061838234902 |
| 171565-4X25ML | 4065268444450 |
| 171565-8L |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.