442879
6-Heptynoic acid
90%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
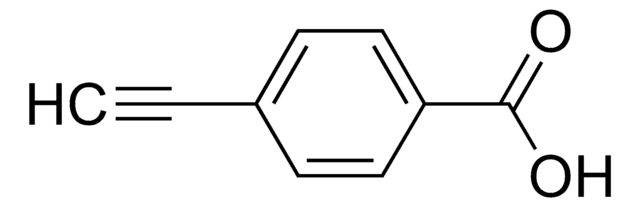
Formula condensata:
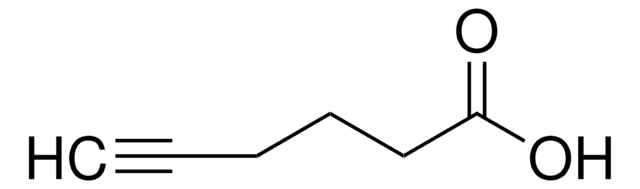
HC≡C(CH2)4COOH
Numero CAS:
Peso molecolare:
126.15
Beilstein:
1747024
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
90%
Indice di rifrazione
n20/D 1.451 (lit.)
P. eboll.
93-94 °C/1 mmHg (lit.)
Densità
0.997 g/mL at 25 °C (lit.)
Gruppo funzionale
carboxylic acid
Stringa SMILE
OC(=O)CCCCC#C
InChI
1S/C7H10O2/c1-2-3-4-5-6-7(8)9/h1H,3-6H2,(H,8,9)
OFCPMJGTZUVUSM-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
6-Heptynoic acid is an alkynoic acid with an acetylene bond. It undergoes condensation with various pyrroles to afford optical diverse fluorescent dyes with a terminal alkyne.
Applicazioni
6-Heptynoic acid may be used for the following syntheses:
- alkyne functionalized Boradiazaindacenes (BODIPY)dyes
- natural products epothilone B and D
- hymenialdisine (HMD) and aldisine (AD) affinity resins
- alkynyl esters
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
235.4 °F - closed cup
Punto d’infiammabilità (°C)
113 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
A novel catalyst with a cuboidal PdMo3S4 core for the cyclization of alkynoic acids to enol lactones.
Wakabayashi T, et al.
Angewandte Chemie (International Edition in English), 35(18), 2123-2124 (1996)
R E Taylor et al.
Organic letters, 3(14), 2221-2224 (2001-07-07)
[reaction: see text] A highly convergent total synthesis of the natural products epothilone B and D is described. The route is highlighted by efficient generation of a C12-C13 trisubstituted olefin which exploits a sequential Nozaki-Hiyama-Kishi coupling and a stereoselective thionyl
Mariano Walter Pertino et al.
Molecules (Basel, Switzerland), 19(2), 2523-2535 (2014-02-26)
Dehydroabietic acid (DHA) is a naturally occurring diterpene with different and relevant biological activities. Previous studies have shown that some DHA derivatives display antiproliferative activity. However, the reported compounds did not include triazole derivatives. Starting from DHA (8,11,13-abietatrien-18-oic acid), and
Martijn Verdoes et al.
Bioorganic & medicinal chemistry letters, 17(22), 6169-6171 (2007-09-25)
The synthesis of three acetylene functionalized BODIPY dyes is described. These dyes are used to fluorescently modify an azido functionalized epoxomicin analogue employing the Huisgen 1,3-dipolar cycloaddition, resulting in a panel of fluorescent epoxomicin derived proteasome probes.
Lauren Ray et al.
Nature communications, 7, 13609-13609 (2016-12-22)
Type I modular polyketide synthases assemble diverse bioactive natural products. Such multienzymes typically use malonyl and methylmalonyl-CoA building blocks for polyketide chain assembly. However, in several cases more exotic alkylmalonyl-CoA extender units are also known to be incorporated. In all
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.