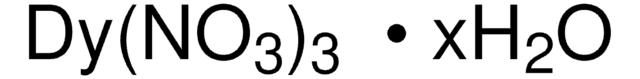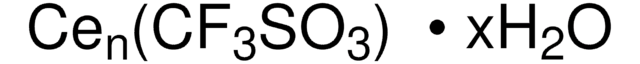425664
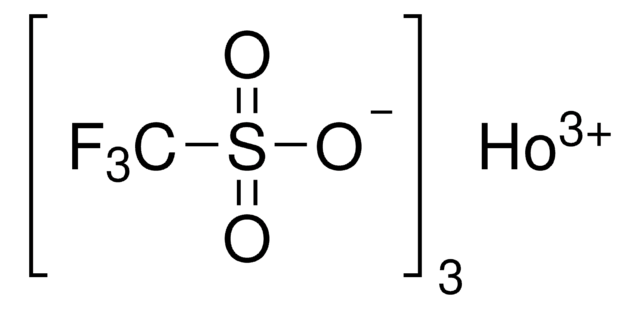
Dysprosium(III) trifluoromethanesulfonate
98%
Sinonimo/i:
Dysprosium(III) triflate, Tris(triflato)dysprosium
About This Item
Prodotti consigliati
Saggio
98%
Impiego in reazioni chimiche
core: dysprosium
reagent type: catalyst
reaction type: Ring-Opening Polymerization
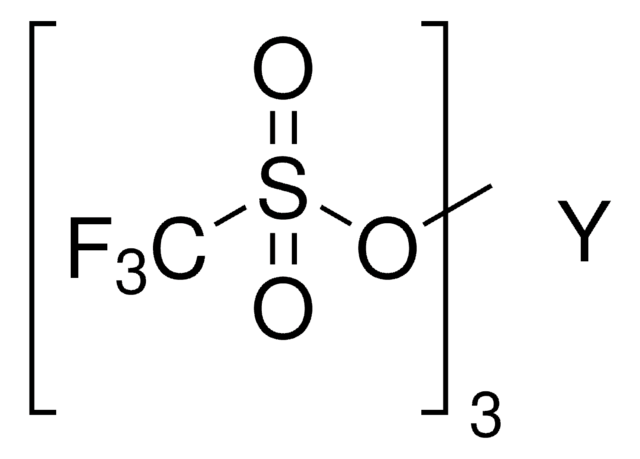
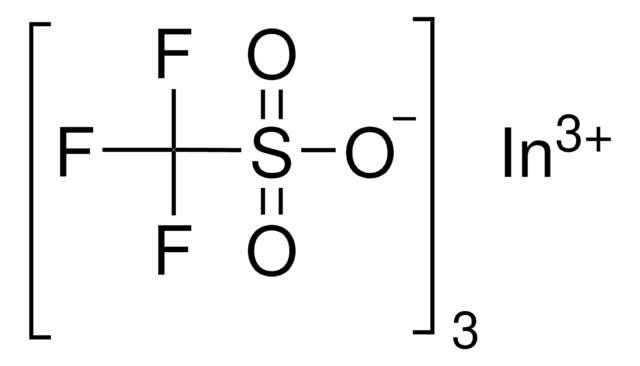
Stringa SMILE
[Dy+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Dy/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
XSVCYDUEICANRJ-UHFFFAOYSA-K
Categorie correlate
Descrizione generale
Applicazioni
- Aza-Piancatelli rearrangement
- Friedel-Crafts alkylation
- Ring-opening polymerization reactions
- Microwave-assisted Kabachnik-Fields condensation
- Cycloaddition reactions (Lewis-acid catalyst)
- Fries rearrangement
- Enantioselective glyoxalate-ene reactions
- Aldol reaction of silyl enol ethers with aldehydes.
- As an effective catalyst for electrophilic substitution reactions of indoles with imines.
- As catalyst for the synthesis of 4-aminocyclopentenones and functionalized azaspirocycles, via intramolecular aza-Piancatelli rearrangement.
- As new curing initiator to study the curing of diglycidyl ether of bisphenol-A (DGEBA).
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.