377198
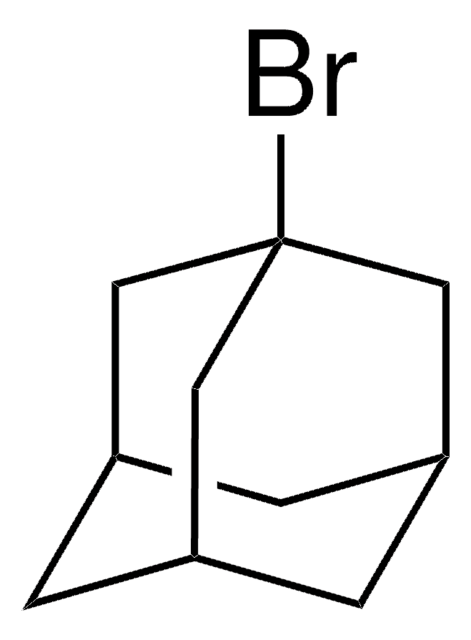
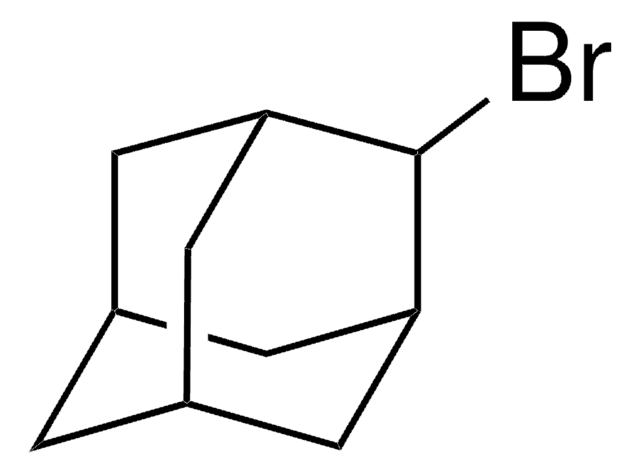
1-Iodoadamantane
98%
Sinonimo/i:
1-Adamantyl iodide, 1-Iodotricyclo[3.3.1.13,7]decane, Adamantyl iodide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H15I
Numero CAS:
Peso molecolare:
262.13
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Forma fisica
solid
Punto di fusione
75-76 °C (lit.)
Stringa SMILE
IC12C[C@H]3C[C@H](C[C@H](C3)C1)C2
InChI
1S/C10H15I/c11-10-4-7-1-8(5-10)3-9(2-7)6-10/h7-9H,1-6H2/t7-,8+,9-,10-
PXVOATXCSSPUEM-CHIWXEEVSA-N
Descrizione generale
1-Iodoadamantane is a haloadamantane. Voltammetric reduction of 1-iodoadamantane at a silver cathode in tetrahydrofuran (THF) and acetonitrile (ACN) is reported to involve a single electron forming a mixture of monomeric and dimeric products. The photoinduced reaction of 1-iodoadamantane in DMSO is reported to afford substitution products on C3, C6, and C8, 1-adamantanol, 1-adamantyl 2-naphthyl ether, and adamantine. The photostimulated reaction of the phthalimide anion with 1-iodoadamantane is reported to yield 3-(1-adamantyl) phthalimide and 4-(1-adamantyl) phthalimide, along with the reduction product adamantane. 1-Iodoadamantane is reported to undergoe photostimulated reaction with the enolate anion of acetone, acetophenone and propiophenone to give admantane and the substitution products.
Applicazioni
1-Iodoadamantane is suitable reagent used to evaluate the rate constants for the reduction of haloadamantanes by SmI2 in presence of hexamethylphosphoramide (HMPA) and H2O by GC/MS-analyzed method. It may be used as iodine atom donor for probing the intermediacy of radical to investigate the chemistry of highly reactive, strained systems such as propellane. It may be used as starting reagent in the synthesis of N-(1-adamantyl)acetamide via nucleophilic substitution. It may be employed in the free-radical carbonylation reactions with alkenes.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Photostimulated reaction of 1-iodoadamantane with carbanionic nucleophiles in DMSO by the SRN1 mechanism.
Borosky GL, et al.
The Journal of Organic Chemistry, 55(12), 3705-3707 (1990)
Juan E Argüello et al.
The Journal of organic chemistry, 68(6), 2362-2368 (2003-03-15)
The fluorescent excited state of the 2-naphthoxide ion (1) is quenched by aliphatic and aromatic halides according to an electron-transfer mechanism, with generation of the corresponding alkyl and aryl radicals by a concerted or consecutive C-X bond fragmentation reaction. Whereas
Dimerization of Cubene. 1-Iodoadamantane as a Probe for Radical Intermediates.
Lukin K and Eaton PE.
Journal of the American Chemical Society, 117(29), 7652-7656 (1995)
Rate study of haloadamantane reduction by samarium diiodide.
Lin T-Y, et al.
J. Chin. Chem. Soc., 49(6), 969-973 (2002)
Christopher A Paddon et al.
Ultrasonics sonochemistry, 14(5), 502-508 (2007-01-17)
The combination of ultrasound and electrochemistry -sonoelectrochemistry can produce a variety of effects within an electrochemical system including enhanced mass transport, in situ cleaning of an electrode surface, diminution of the diffusion layer, and possible induction of new reactions by
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.