346225
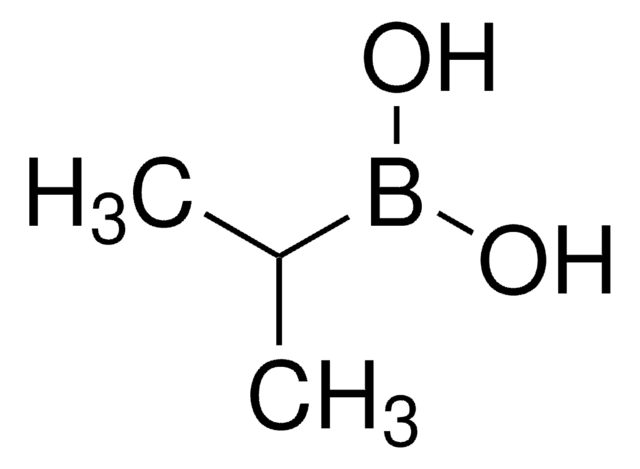
(2-Methylpropyl)boronic acid
≥95.0%
Sinonimo/i:
Isobutaneboronic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(CH3)2CHCH2B(OH)2
Numero CAS:
Peso molecolare:
101.94
Numero MDL:
Codice UNSPSC:
12352103
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥95.0%
Stato
solid
Punto di fusione
108-111 °C (lit.)
Stringa SMILE
CC(C)CB(O)O
InChI
1S/C4H11BO2/c1-4(2)3-5(6)7/h4,6-7H,3H2,1-2H3
ZAZPDOYUCVFPOI-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
(2-Methylpropyl)boronic acid can be used as:
It can also be used as a reactant in:
- A reactant in the preparation of 4-isobutylisoquinoline from 4-bromoisoquinoline by Suzuki-Miyaura type couple reaction.
- A catalyst along with aluminum hydroxide, boric acid in the polymerization of styrene.
It can also be used as a reactant in:
- Copper catalyzed cross-coupling reactions.
- The synthesis of polyborylalkanes by Ir-catalyzed C-H borylation reaction.
- The preparation of heterosubstituted diazaboroles and borinines.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Study of Rate-accelerating of Aluminum Hydroxide, Boric Acid, and (2-Methylpropyl) Boronic Acid for Atom Transfer Radical Polymerization of Styrene
Luo Yu-tai, et al.
Journal of Xiamen University (Natural Science), 47(1), 63-63 (2008)
Steven A Rossi et al.
Organic letters, 15(9), 2314-2317 (2013-04-25)
For the first time, a general catalytic procedure for the cross-coupling of primary amides and alkylboronic acids is demonstrated. The key to the success of this reaction was the identification of a mild base (NaOSiMe3) and oxidant (di-tert-butyl peroxide) to
Takeshi Yamamoto et al.
Organic letters, 21(16), 6235-6240 (2019-08-07)
Pyrazolylaniline serves as a temporary directing group attached to the boron atom of alkylboronic acids in Ir-catalyzed C(sp3)-H borylation. The reaction takes place at α-, β-, and γ-C-H bonds, giving polyborylated products including di-, tri-, tetra-, and even pentaborylalkanes. α-C-H
Benjamin M Reeves et al.
Angewandte Chemie (International ed. in English), 58(44), 15697-15701 (2019-09-06)
A transition-metal-free reductive hydroxymethylation reaction has been developed, enabling the preparation of tetrahydroisoquinolines bearing C4-quaternary centers from the corresponding isoquinolines. Deuterium labelling studies and control experiments enable a potential mechanism to be elucidated which features a key Cannizzaro-type reduction followed
Solution-state 15N NMR and solid-state single-crystal XRD study of heterosubstituted diazaboroles and borinines prepared via an effective and simple microwave-assisted solvent-free synthesis
Slabber CA, et al.
Journal of Organometallic Chemistry, 723, 122-128 (2013)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.