456772
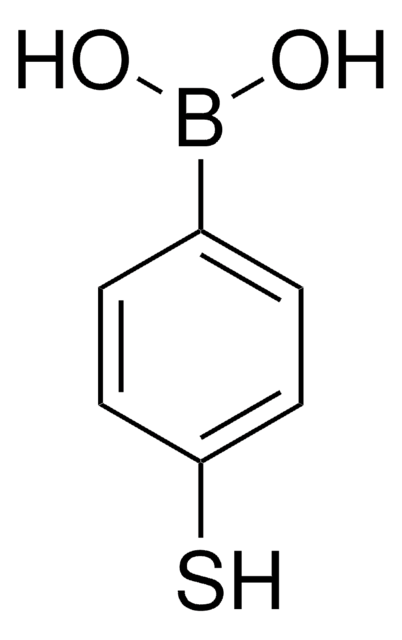
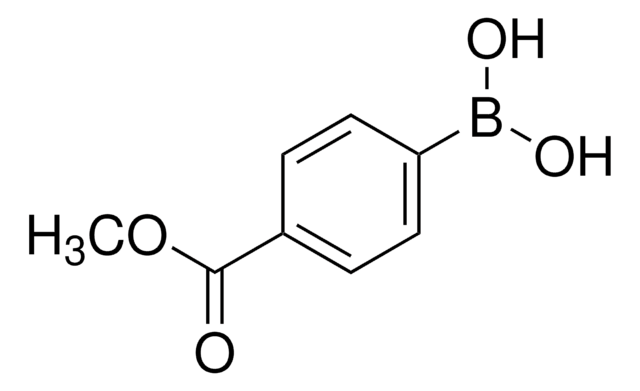
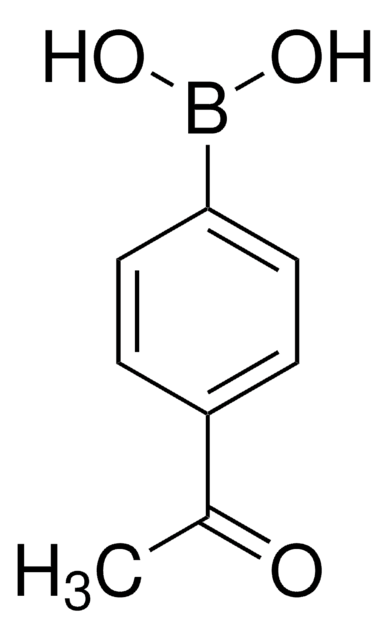
4-Carboxyphenylboronic acid
Sinonimo/i:
4-(Dihydroxyboronyl)benzoic acid, 4-(Dihydroxyboryl)benzoic acid, 4-Boronobenzoic acid, 4-Carboxybenzeneboronic acid, 4-Carboxylphenylboronic acid, 4-Hydroxycarbonylphenyl boronic acid, NSC 221170, p-Boronobenzoic acid, p-Carboxybenzeneboronic acid, p-Carboxyphenylboronic acid
About This Item
Prodotti consigliati
Livello qualitativo
Punto di fusione
220 °C (dec.) (lit.)
Stringa SMILE
OB(O)c1ccc(cc1)C(O)=O
InChI
1S/C7H7BO4/c9-7(10)5-1-3-6(4-2-5)8(11)12/h1-4,11-12H,(H,9,10)
SIAVMDKGVRXFAX-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Condensation reactions with stabilizer chains at the surface of polystyrene latex
- Suzuki coupling reactions
- Esterification
- Derivatization of polyvinylamine
- Synthesis of isotopically labeled mercury
- Functionalization of poly-SiNW for detection of dopamine
- Suzuki-Miyaura cross-coupling
- Induction of pH sensitivity on fluorescence lifetime of quantum dots by NIR fluorescent dyes
- Bio-supported palladium nanoparticles as phosphine-free catalyst for Suzuki reaction in water
- Chan-Lam-type Copper (Cu)-catalyzed S-arylation with aryl boronic acids at room temperature
Reagent used in Preparation of
- Isoquinolones via regioselective Suzuki-Miyaura cross-coupling and tandem palladium-catalyzed intramolecular aminocarbonylation and annulation
- Amprenavir-based P1-substituted bi-aryl derivatives as ultra-potent HIV-1 protease inhibitors
- Phenols via visible-light initiated aerobic oxidative hydroxylation of arylboronic acids using air as oxidant catalyzed by Ruthenium (Ru)-complex
- Glucose sensitive boronic acid-bearing block copolymers
- Trisulfonated calixarene upper-rim sulfonamido derivatives and their complexation with the trimethyllysine epigenetic mark
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.