470821
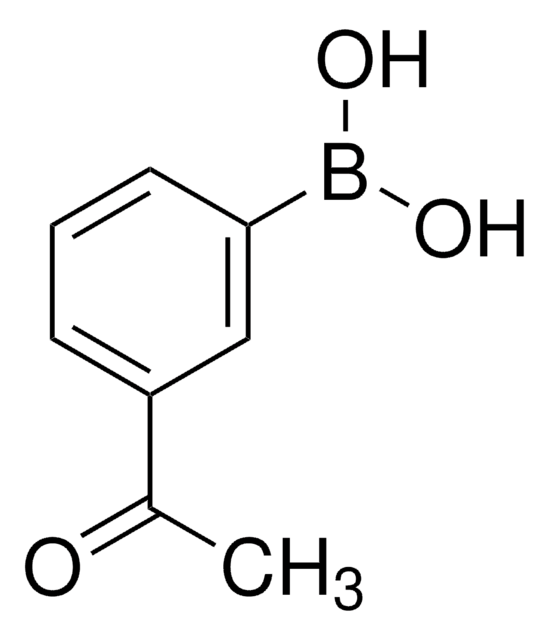
4-Acetylphenylboronic acid
95%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
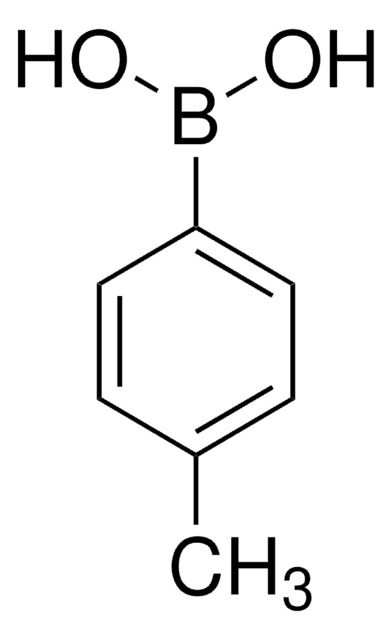
Formula condensata:
CH3COC6H4B(OH)2
Numero CAS:
Peso molecolare:
163.97
Numero MDL:
Codice UNSPSC:
12352103
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
95%
Punto di fusione
240-244 °C (lit.)
Gruppo funzionale
ketone
Stringa SMILE
CC(=O)c1ccc(cc1)B(O)O
InChI
1S/C8H9BO3/c1-6(10)7-2-4-8(5-3-7)9(11)12/h2-5,11-12H,1H3
OBQRODBYVNIZJU-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
4-Acetylphenylboronic acid is a boronate, belongs to a class of synthetic organic compounds. It reacts rapidly with peroxynitrite (ONOO(-)) to form stable hydroxy derivatives. It undergoes Suzuki coupling with 4-bromotriphenylamine catalyzed by dichlorobis(triphenylphosphine)Pd(II), during the synthesis of dendrimers.
Applicazioni
4-Acetylphenylboronic acid was used in the synthesis of 4′-azidoacetophenone.
Reactant involved in:
- Palladium-catalyzed decarboxylative coupling
- Copper-catalyzed hydroxylation
- Palladium-catalyzed Suzuki-Miyaura cross-coupling
- Cross-coupling with α-bromocarbonyl compounds
- Oxidation catalyzed by Baeyer-Villiger monooxygenases
- 1,5-substitution reactions
Altre note
Contains varying amounts of anhydride
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Kimberly D Grimes et al.
Synthesis, 2010(9), 1441-1448 (2010-06-08)
We report the copper(II)-catalyzed conversion of organoboron compounds into the corresponding azide derivatives. A systematic series of phenylboronic acid derivatives is evaluated to examine the importance of steric and electronic effects of the substituents on reaction yield as well as
Adam Sikora et al.
Free radical biology & medicine, 47(10), 1401-1407 (2009-08-19)
In this study, we show that boronates, a class of synthetic organic compounds, react rapidly and stoichiometrically with peroxynitrite (ONOO(-)) to form stable hydroxy derivatives as major products. Using a stopped-flow kinetic technique, we measured the second-order rate constants for
A New Efficient Convergent Synthesis of Conjugated Aryl-containing Dendrimers.
El-Deeb IM and Lee SH.
Bull. Korean Chem. Soc., 31(6), 1757-1760 (2010)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)

![1-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]ethanone AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/280/787/64aa2a50-1d44-4c16-ace9-c54ea40606e6/640/64aa2a50-1d44-4c16-ace9-c54ea40606e6.png)