303143
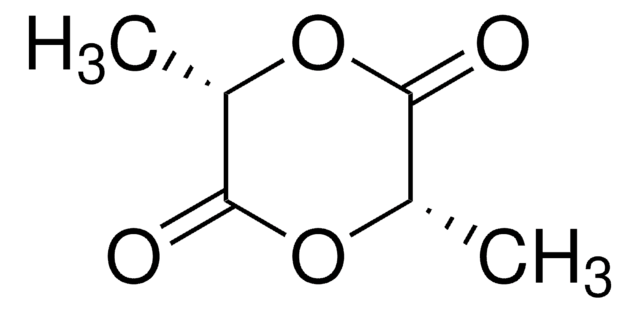
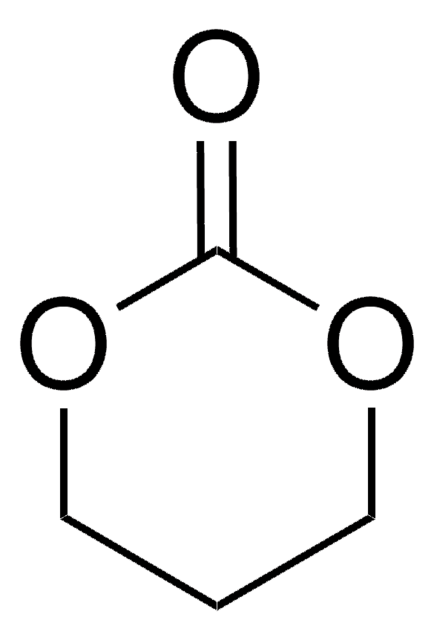
3,6-Dimethyl-1,4-dioxane-2,5-dione
99%
Sinonimo/i:
DL-Lactide, rac-Lactide, Lactide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C6H8O4
Numero CAS:
Peso molecolare:
144.13
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
99%
Forma fisica
crystals
P. eboll.
142 °C/8 mmHg (lit.)
Punto di fusione
116-119 °C
Temperatura di conservazione
2-8°C
Stringa SMILE
CC1OC(=O)C(C)OC1=O
InChI
1S/C6H8O4/c1-3-5(7)10-4(2)6(8)9-3/h3-4H,1-2H3
JJTUDXZGHPGLLC-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
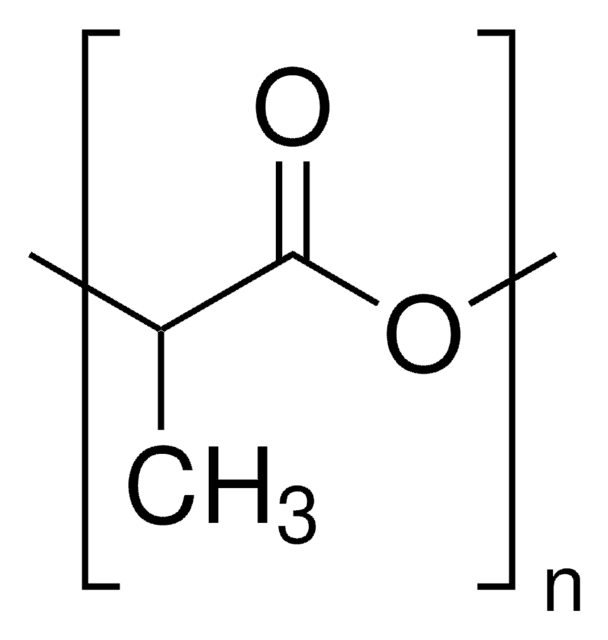
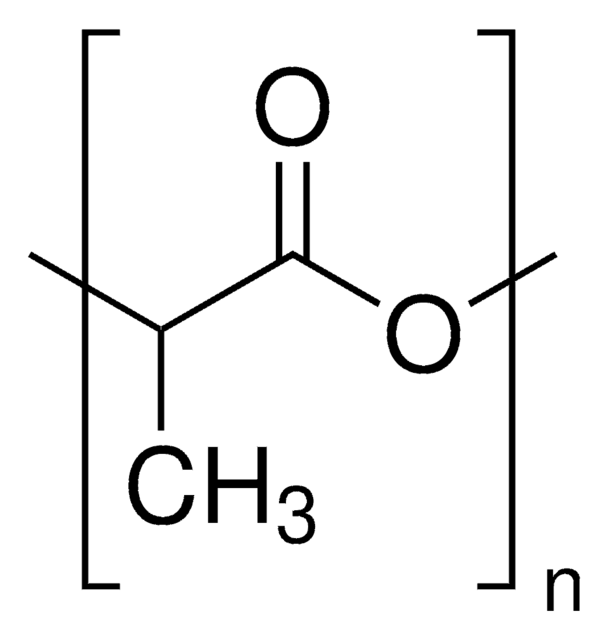
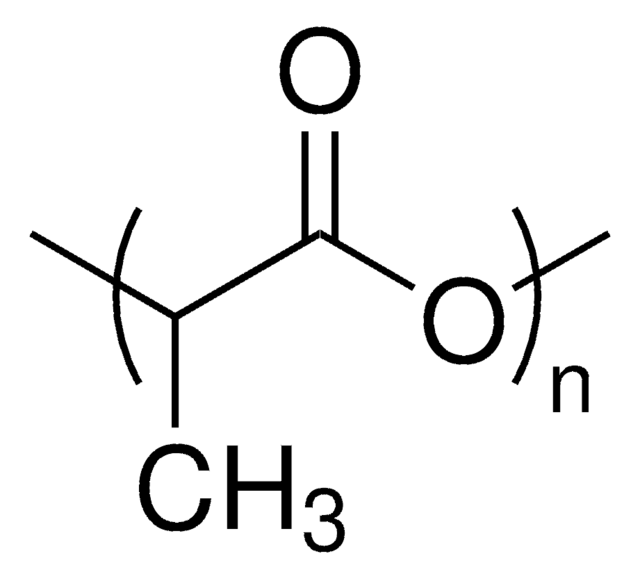
3,6-Dimethyl-1,4-dioxane-2,5-dione (or rac-lactide), is the 50:50 racemic mixture of D- and L-Lactide. Rac-lactide is a lactone derived from lactic acid that has attracted great interest in academia and commercial applications, as it is derived from abundant renewable resources. Rac-lactide can be ready polymerized via ring-opening polymerization, using a variety of metal or organocatalysts, yielding poly(D,L-lactide). While the resulting polymer is generally amorphous, the use of stereospecific catalysts can lead to heterotactic PLA, which exhibits some degree of crystallinity.
Applicazioni
3,6-Dimethyl-1,4-dioxane-2,5-dione can be used as a reactant:
- To synthesize multi-block copolymers of polylactide and polycarbonate.
- In the aluminum-catalyzed polymerization of propene oxide, lactide, and phthalic anhydride to produce multi-block polyesters.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Insun Yu et al.
Journal of the American Chemical Society, 134(30), 12758-12773 (2012-07-07)
A family of racemic and enantiopure indium complexes 1-11 bearing bulky chiral diaminoaryloxy ligands, H(NNO(R)), were synthesized and fully characterized. Investigation of both the mono- and the bis-alkoxy-bridged complexes [(NNO(R))InX](2)[μ-Y][μ-OEt] (5, R = (t)Bu, X = Y = Cl; 8
Kimberly M Osten et al.
Dalton transactions (Cambridge, England : 2003), 41(26), 8123-8134 (2012-04-07)
Functionalized diaminophenols, H(N(R1R2)N(R3)O), were investigated as ligands for indium catalysts in the ring-opening polymerization of racemic lactide. Precursor complexes (N(Me2)N(Me)O)InCl(2) (1), (N(Pr2)NO)InCl(2) (2), and (N(Mes)NO)InCl(2) (3) were synthesized and fully characterized by (1)H and (13)C NMR spectroscopy, elemental analysis, and
Vibrational Optical Activity of (3 S, 6 S)-3, 6-Dimethyl-1, 4-dioxane-2, 5-dione.
Tam CN, et al.
Journal of the American Chemical Society, 118(42), 10285-10293 (1996)
Fabio Marchetti et al.
Dalton transactions (Cambridge, England : 2003), 42(8), 2792-2802 (2012-09-11)
A series of group 4 metal tetracarbamates M(O(2)CNR(2))(4) (M = Ti, R = Et, 1a; M = Zr, R = Et, 1b; (i)Pr, 1c; M = Hf, R = Et, 1d; R = (i)Pr, 1e) were studied as catalytic precursors
Lu Qin et al.
Dalton transactions (Cambridge, England : 2003), 48(32), 12315-12325 (2019-07-26)
In this paper we report a series of Al(iii) complexes supported by N,O-bidentate β-pyrazyl functionalized enolate ligands HL1-HL5 (L = (6-Me-2,5-C4H2N2)-CH[double bond, length as m-dash]C(R)-O-), (R = tBu, Ph, p-tolyl, p-OMePh, o-tolyl) and their exploitation for the ring-opening polymerization of
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.