214671
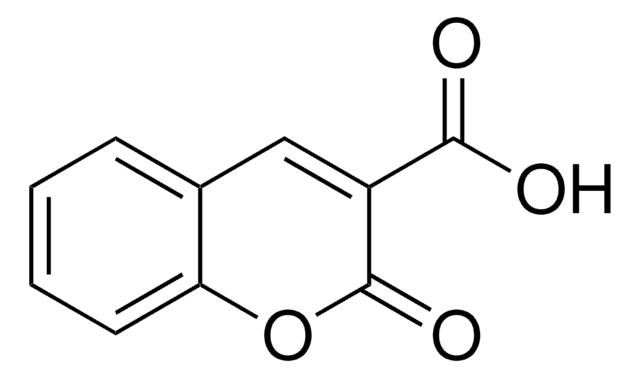
3-Acetylcoumarin
96%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C11H8O3
Numero CAS:
Peso molecolare:
188.18
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
96%
Stato
solid
Punto di fusione
119-122 °C (lit.)
Gruppo funzionale
ester
ketone
Stringa SMILE
CC(=O)C1=Cc2ccccc2OC1=O
InChI
1S/C11H8O3/c1-7(12)9-6-8-4-2-3-5-10(8)14-11(9)13/h2-6H,1H3
CSPIFKKOBWYOEX-UHFFFAOYSA-N
Descrizione generale
FTIR and FT-Raman spectra of 3-acetylcoumarin has been reported. 3-Acetylcoumarin undergoes condensation with aryl aldehydes in chloroform in the presence of piperidine to yield coumarin derivatives containing 4-arylbut-3-en-2-one moiety.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Anuradha Ramoji et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 68(3), 504-509 (2007-03-03)
Laser Raman (3500-50 cm(-1)) and IR (4000-400 cm(-1)) spectral measurements have been made on the laboratory prepared solid 3-acetylcoumarin. Molecular electronic energy, equilibrium geometrical structure and harmonic vibrational spectra have been computed at the RHF/6-31G(d,p) and B3LYP/6-31G(d,p) levels of theory.
Puja Kapoor et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 83(1), 74-81 (2011-09-10)
The present work stems from our interest in the synthesis, characterization and biological evaluation of lanthanide(III) complexes of a class of coumarin based imines which have been prepared by the interaction of hydrated lanthanide(III) chloride with the sodium salts of
Rekha S Hunoor et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 77(4), 838-844 (2010-09-14)
Co(II), Ni(II), Cu(II) and Zn(II) complexes with a new heterocyclic Schiff base derived by the condensation of isonicotinoylhydrazide and 3-acetylcoumarin have been synthesized. ¹H, ¹³C and 2D HETCOR NMR analyses confirm the formation of title compound and existence of the
Abdullah Sulaiman Al-Ayed
Molecules (Basel, Switzerland), 16(12), 10292-10302 (2011-12-14)
A series of new coumarin derivatives 4 containing a 4-arylbut-3-en-2-one moiety were synthesized by condensation of 3-acetylcoumarin 1 with aryl aldehydes 2 in chloroform in the presence of piperidine. The interactions of 3-formyl-4-chlorocoumarin (3) with nitrogen-containg nucleophiles leading to the
M Takeshita et al.
Research communications in molecular pathology and pharmacology, 88(1), 123-126 (1995-04-01)
In the incubation of 3-acetylcoumarin in rat liver supernatant fraction (S-9), four metabolites were isolated, and two of which were inseparable diastereomeric mixture as a major product. However, when 3-acetylcoumarin was fermented with baker's yeast (Saccharomyces cerevisiae), only two compounds
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.