19240
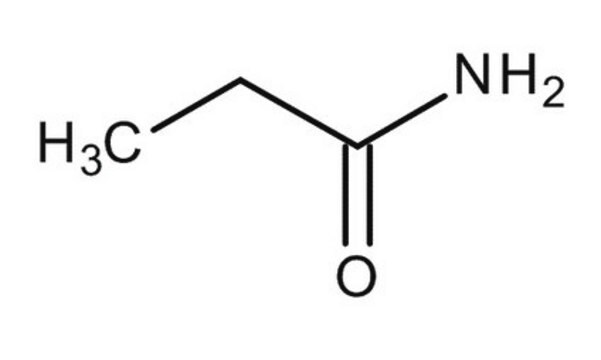
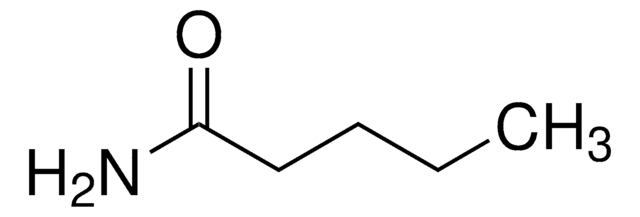
Butyramide
≥98.0% (T)
Sinonimo/i:
Amide C4
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
CH3CH2CH2CONH2
Numero CAS:
Peso molecolare:
87.12
Beilstein:
1361528
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
≥98.0% (T)
Punto di fusione
114-116 °C
Solubilità
alcohol: soluble(lit.)
diethyl ether: slightly soluble(lit.)
water: soluble(lit.)
Gruppo funzionale
amide
Stringa SMILE
CCCC(N)=O
InChI
1S/C4H9NO/c1-2-3-4(5)6/h2-3H2,1H3,(H2,5,6)
DNSISZSEWVHGLH-UHFFFAOYSA-N
Informazioni sul gene
rat ... Ggt1(116568)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
Butyramide was used in the synthesis of hydroxamic acids, electrorheological fluids and β-amodoorganotin compounds. It was used as substrate of (+)-γ-lactamase to develop a microreactor to study enzyme stability, activity, kinetics and substrate specificity.
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Hongjian Zhang et al.
Drug metabolism and disposition: the biological fate of chemicals, 35(5), 795-805 (2007-02-17)
2-{Butyryl-[2'-(4,5-dimethyl-isoxazol-3-ylsulfamoyl)-biphenyl-4-ylmethyl]-amino}-N-isopropyl-3-methyl-butyramide (BMS-1) is a potent dual acting angiotensin-1 and endothelin-A receptor antagonist. The compound was subject to rapid metabolic clearance in monkey and human liver microsomes and exhibited low systemic exposure and marked interanimal variability in cynomolgus monkeys after p.o.
Effect of butyrate analogues on proliferation and differentiation in human neuroblastoma cell lines.
P Rocchi et al.
Anticancer research, 18(2A), 1099-1103 (1998-06-06)
Butyric acid has been shown in vitro to produce cytodifferentiation of a wide variety of neoplastic cells. The potential clinical use of this compound as a therapeutic agent is limited by its rapid metabolism. This has led to the examination
B P O'Hara et al.
Protein engineering, 13(2), 129-132 (2000-03-10)
The AmiC protein in Pseudomonas aeruginosa is the negative regulator and ligand receptor for an amide-inducible aliphatic amidase operon. In the wild-type PAC1 strain, amidase expression is induced by acetamide or lactamide, but not by butyramide. A mutant strain of
Rajendra Singh et al.
Bioprocess and biosystems engineering, 41(8), 1225-1232 (2018-05-12)
Butyramide is a commodity chemical having wide range of applications from material science to biological sciences including synthesis of therapeutic drugs, hydroxamic acids, and electrorheological fluids. The nitrile hydratase protein of Bacillus sp. APB-6 was explored to develop an efficient
R A Norman et al.
The Journal of biological chemistry, 275(39), 30660-30667 (2000-07-13)
Expression of the amidase operon of Pseudomonas aeruginosa is controlled by AmiC, the ligand sensor and negative regulator, and AmiR the transcription antitermination factor activator. We have titrated out AmiC repression activity in vivo by increased AmiR production in trans
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.