M26305
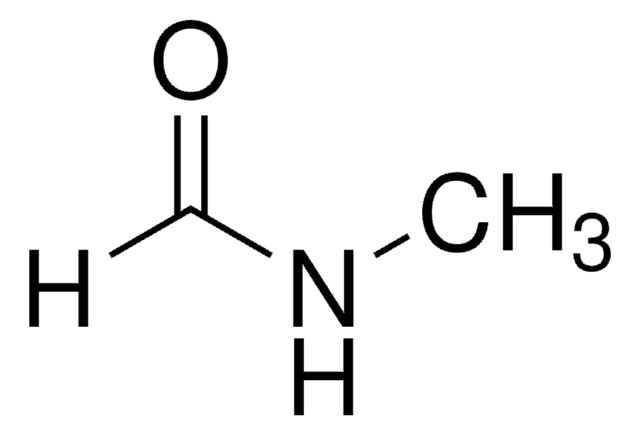
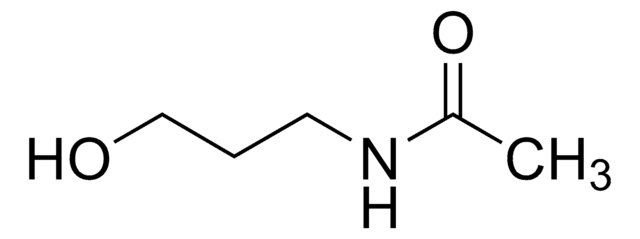
N-Methylacetamide
≥99%
Sinonimo/i:
Acetylmethylamine
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥99%
Indice di rifrazione
n20/D 1.433 (lit.)
P. eboll.
204-206 °C (lit.)
Punto di fusione
26-28 °C (lit.)
Densità
0.957 g/mL at 25 °C (lit.)
Stringa SMILE
CNC(C)=O
InChI
1S/C3H7NO/c1-3(5)4-2/h1-2H3,(H,4,5)
OHLUUHNLEMFGTQ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- To synthesize N-methyl-N-(3-thienyl)acetamide by reacting with 3-bromothiophene in the presence of CuI catalyst and N,N′-dimethylethylenediamine.(1)
- As a ligand to synthesize the zirconium(IV) complex, Zr(MeC(O)NMe)4 by reacting with tetrakis(dimethylamido)zirconium.(2)
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Repr. 1B
Codice della classe di stoccaggio
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
240.8 °F
Punto d’infiammabilità (°C)
116 °C
Dispositivi di protezione individuale
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Elenchi normativi
Forniamo informazioni su eventuali restrizioni prevalentemente per i prodotti chimici. Per altre tipologie di prodotto siamo in grado di fornire soltanto informazioni limitate. Nessuna segnalazione significa che nessuno dei componenti è citato in un elenco. È dovere dell’utilizzatore assicurarsi che il prodotto venga impiegato in maniera sicura e a norme di legge.
EU REACH SVHC Candidate List
EU REACH Annex XVII (Restriction List)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.