177989
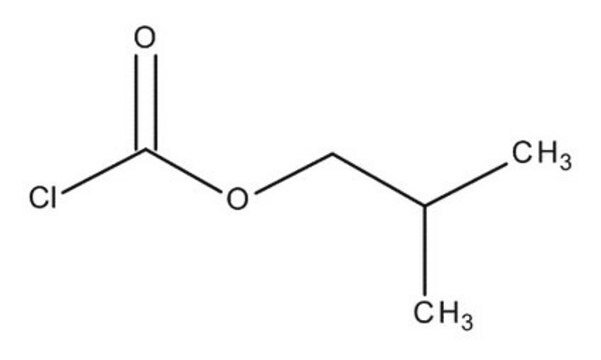
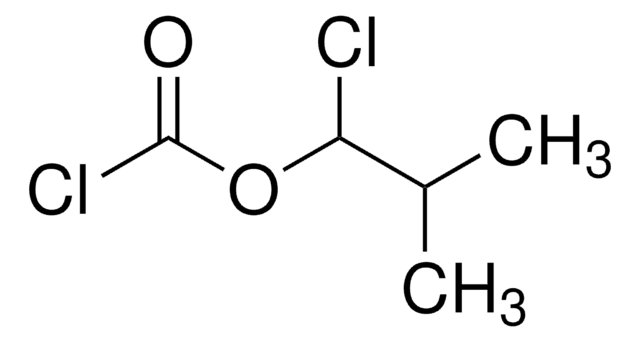
Isobutyl chloroformate
98%
Sinonimo/i:
Chloroformic acid isobutyl ester, IBCF
About This Item
Prodotti consigliati
Tensione di vapore
0.33 mmHg ( 20 °C)
Livello qualitativo
Saggio
98%
Forma fisica
liquid
Indice di rifrazione
n20/D 1.407 (lit.)
P. eboll.
128.8 °C (lit.)
Solubilità
benzene: miscible
chloroform: miscible
diethyl ether: miscible
Densità
1.053 g/mL at 25 °C (lit.)
Gruppo funzionale
chloro
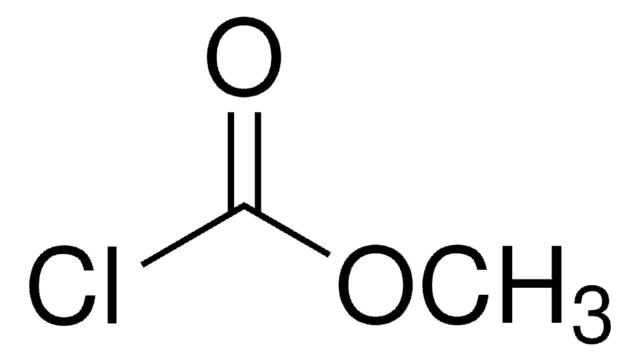
Stringa SMILE
CC(C)COC(Cl)=O
InChI
1S/C5H9ClO2/c1-4(2)3-8-5(6)7/h4H,3H2,1-2H3
YOETUEMZNOLGDB-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Preparation of a volatile derivative of taurine and application to gas chromatographic determination of urinary taurine.: Demonstrates the utility of isobutyl chloroformate in preparing volatile derivatives of taurine for gas chromatographic analysis, offering a methodological advancement in clinical biochemistry (Masuoka et al., 1989).
- Quinazoline antifolates inhibiting thymidylate synthase: synthesis of four oligo(L-gamma-glutamyl) conjugates of N10-propargyl-5,8-dideazafolic acid and their enzyme inhibition.: This article investigates the synthesis and biological activity of quinazoline antifolates, using techniques including isobutyl chloroformate, relevant in medicinal chemistry and drug development (Pawelczak et al., 1989).
- Coupling of peptides to protein carriers by mixed anhydride procedure.: Discusses a novel technique using isobutyl chloroformate for peptide coupling to proteins, useful in bioconjugate chemistry and vaccine development (Samokhin & Filimonov, 1985).
- Folate analogues altered in the C9-N10 bridge region. 18. Synthesis and antitumor evaluation of 11-oxahomoaminopterin and related compounds.: Investigates the role of isobutyl chloroformate in synthesizing folate analogues for cancer research, showing its importance in therapeutic chemistry (Nair et al., 1981).
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
96.8 °F - closed cup
Punto d’infiammabilità (°C)
36 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.