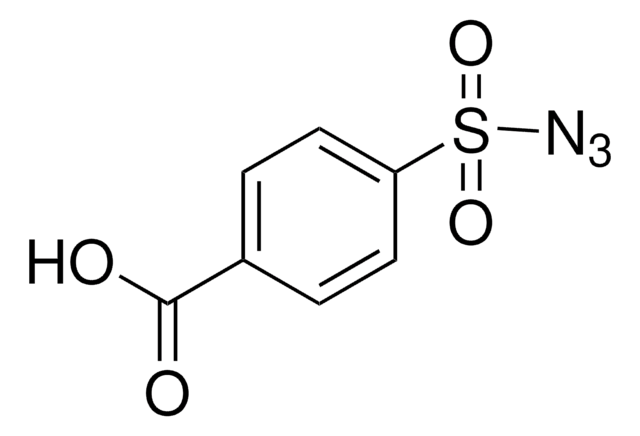
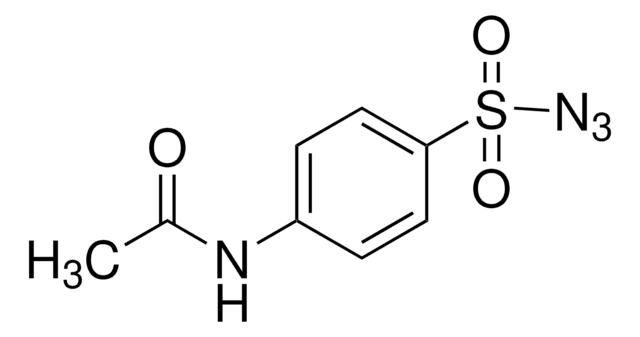
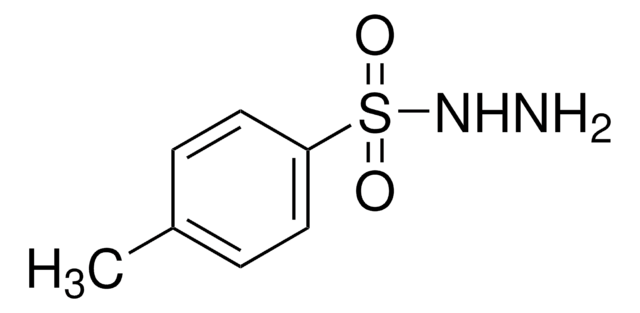
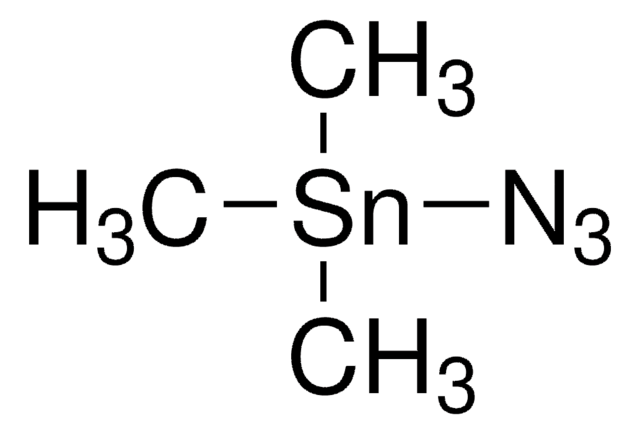155071
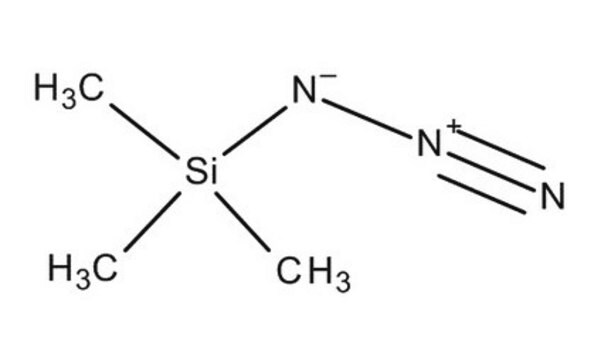
Azidotrimethylsilane
95%
Sinonimo/i:
Trimethylsilyl azide
About This Item
Prodotti consigliati
Saggio
95%
Stato
liquid
Indice di rifrazione
n20/D 1.414 (lit.)
P. ebollizione
52-53 °C/175 mmHg (lit.)
Densità
0.868 g/mL at 25 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
C[Si](C)(C)N=[N+]=[N-]
InChI
1S/C3H9N3Si/c1-7(2,3)6-5-4/h1-3H3
SEDZOYHHAIAQIW-UHFFFAOYSA-N
Descrizione generale
Applicazioni
- A nitrogen precursor to prepare GaN nanowire via metal-organic chemical vapor deposition method.
- An electrolyte additive in Li-O2 batteries. The addition of TMSN3 results in the formation of robust solid electrolyte interphase.
- An efficient reagent in the synthesis of tetrazoles, fullerenyl azide, and α-azido oximes.
- A silylating agent in the O-trimethyl silylation of alcohols and phenols.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
42.8 °F - closed cup
Punto d’infiammabilità (°C)
6 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
“Click” chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
The chemistry of organoazides is exceedingly rich, since the azide functionality reacts with electrophiles, nucleophiles, and dipolarophiles, with or without the extrusion of dinitrogen. Common place transformation such as Staudinger reductions or ligations, Cu(I)-catalyzed Huisgen cycloadditions (of the “click” reaction family), Curtius or Schmidt rearrangents, nitrene reactions, or imine formation via aza-Wittig reactions all necessitate organoazide precursors or intermediates
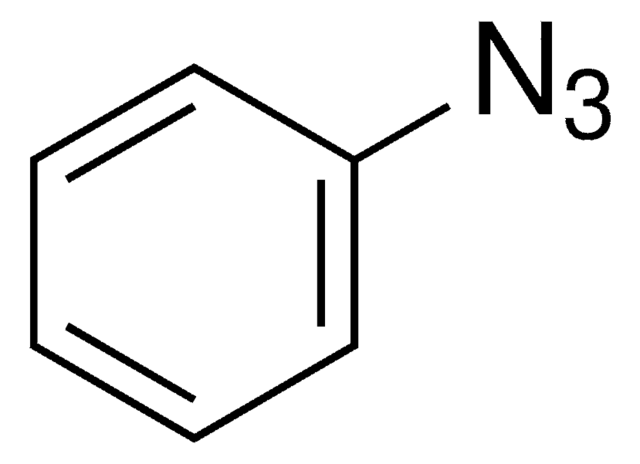
Since the preparation of the first organic azide, phenyl azide, by Peter Griess in 1864 this energy-rich and versatile class of compounds has enjoyed considerable interest.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.