142662
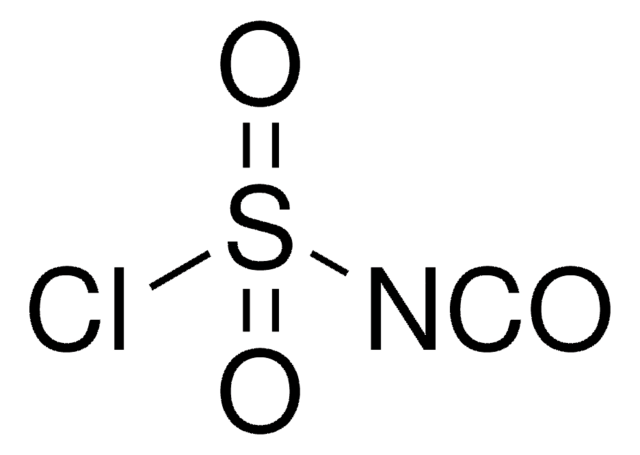
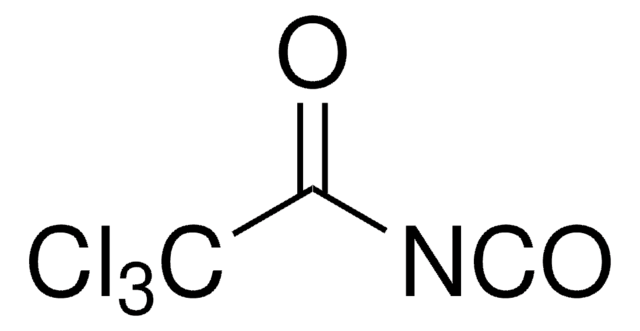
Chlorosulfonyl isocyanate
98%
Sinonimo/i:
CSI
About This Item
Prodotti consigliati
Tensione di vapore
5.57 psi ( 20 °C)
Livello qualitativo
Saggio
98%
Stato
liquid
Indice di rifrazione
n20/D 1.447 (lit.)
P. ebollizione
107 °C (lit.)
Punto di fusione
−44 °C (lit.)
Densità
1.626 g/mL at 25 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
ClS(=O)(=O)N=C=O
InChI
1S/CClNO3S/c2-7(5,6)3-1-4
WRJWRGBVPUUDLA-UHFFFAOYSA-N
Informazioni sul gene
human ... ABCB1(5243) , FPR1(2357)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Chlorosulfonyl isocyanate was used in synthesis and biochemical characterization of new class of membrane-associated glycosyltransferase inhibitors.
- It is synthetically versatile reagent and was used in the preparation of amides, lactams and triazocinones.
- It was employed in regio- and diastereoselective introduction of a protected amino group in synthesis of chiral, polyhydroxylated piperidines.
- It was used as reagent during the synthesis of novel sulfamides.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
Rischi supp
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
>230.0 °F
Punto d’infiammabilità (°C)
> 110 °C
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Protocolli
To optimize hydrolysis using β-glucuronidase, factors such as incubation time, temperature, hydrolysis pH, enzyme source, and enzyme concentration must be evaluated for each glucuronide metabolite to be analyzed.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.