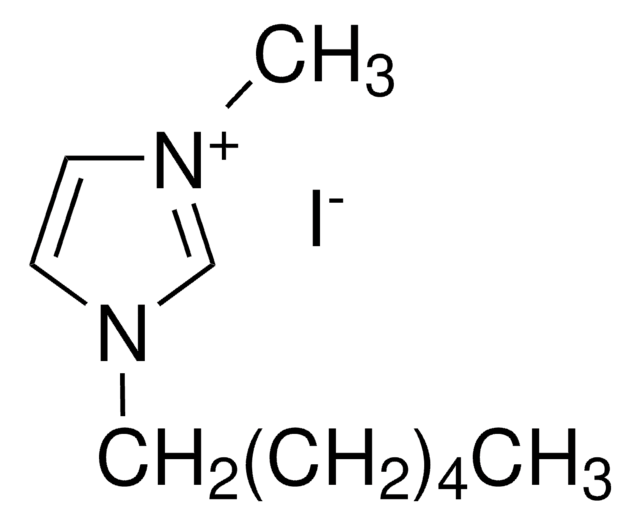
140775
Tetrabutylammonium iodide
reagent grade, 98%
Sinonimo/i:
TBAI
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(CH3CH2CH2CH2)4N(I)
Numero CAS:
Peso molecolare:
369.37
Beilstein:
3916152
Numero CE:
Numero MDL:
Codice UNSPSC:
12352107
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Grado
reagent grade
Livello qualitativo
Saggio
98%
Stato
solid
Punto di fusione
141-143 °C (lit.)
Stringa SMILE
[I-].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.HI/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h5-16H2,1-4H3;1H/q+1;/p-1
DPKBAXPHAYBPRL-UHFFFAOYSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
Tetrabutylammonium iodide (TBAI) has been used as a catalyst in the following reactions:
Other reactions where TBAI can be used as a catalyst:
- Synthesis of O-benzyl-N-Boc-L-tyrosine benzyl ester from N-Boc-L-tyrosine.
- Conversion of 8-fluoro-1-aminonaphthalene into 1-(8-fluoro-naphthalen-1-yl)piperazine hydrochloride.
- Synthesis of 1-(2,4-dichlorophenyl)-5-(4-(4-iodobut-1-ynyl)phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide from 4-(4-(1-(2,4-dichlorophenyl)-4-methyl-3-(piperidin-1-ylcarbamoyl)-1H-pyrazol-5-yl)phenyl)but-3-yn-1-yl methanesulfonate.
Other reactions where TBAI can be used as a catalyst:
- TBAI-tert-butyl hydroperoxide system can catalyze the conversion of α-methyl styrene derivatives into allylic sulfones by reacting with sulfonylhydrazides under metal-free conditions.
- Palladium(0)-catalyzed cross-coupling between benzylic zinc bromides and aryl or alkenyl triflates.
- Three-component coupling of amines, carbon dioxide, and halides to form carbamates in the presence of cesium carbonate.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
New Efficient Nickel-and Palladium-Catalyzed Cross-Coupling Reactions Mediated by Tetrabutylammonium Iodide.
Piber M,et al.
Organic Letters, 9(1), 1323-1326 (1999)
Efficient carbamate synthesis via a three-component coupling of an amine, CO2, and alkyl halides in the presence of Cs2CO3 and tetrabutylammonium iodide.
Salvatore R N, et al.
The Journal of Organic Chemistry, 66(3), 1035-1037 (2001)
Solid-phase asymmetric synthesis using a polymer-supported chiral Evans'-type oxazolidin-2-one.
Green R, et al.
Nature Protocols, 8(10), 1890-1890 (2013)
Tetrabutylammonium iodide catalyzed allylic sulfonylation of α-methyl styrene derivatives with sulfonylhydrazides.
Li X Xu X and Zhou C
Chemical Communications (Cambridge, England), 48(100), 12240-12242 (2012)
Crystal structure of the human cannabinoid receptor CB 1.
Hua T, et al.
Cell, 167(3), 750-762 (2016)
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 140775-10KG | |
| 140775-100G | 4061838733672 |
| 140775-500G | 4061838733696 |
| 140775-25G | 4061838733689 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.