129542
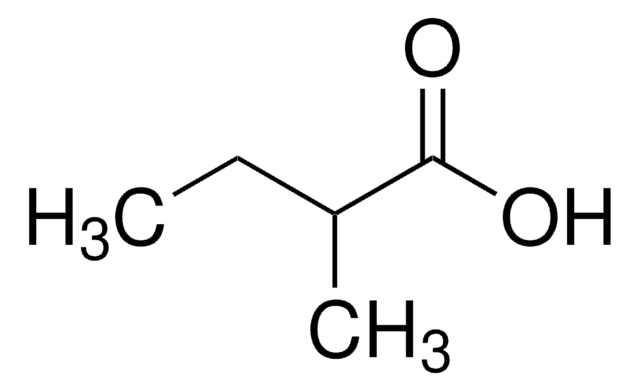
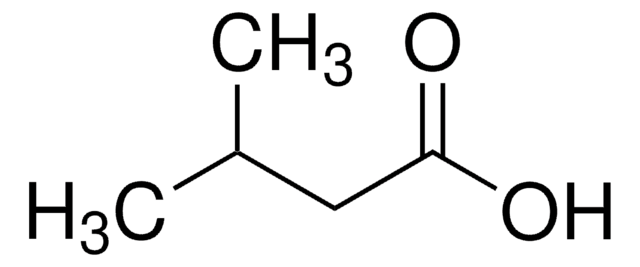
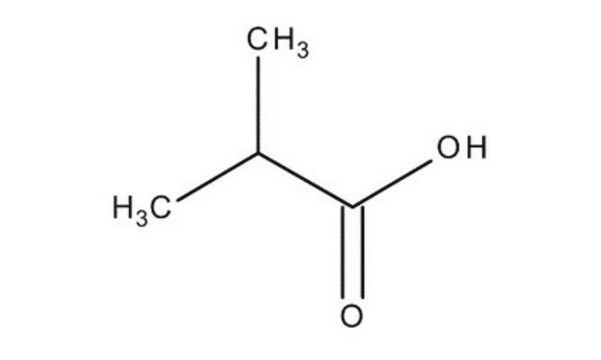
Isovaleric acid
99%
Sinonimo/i:
3-Methylbutanoic acid, 3-Methylbutyric acid
About This Item
Prodotti consigliati
Tensione di vapore
0.38 mmHg ( 20 °C)
Livello qualitativo
Saggio
99%
Stato
liquid
Temp. autoaccensione
824 °F
Indice di rifrazione
n20/D 1.403 (lit.)
P. ebollizione
175-177 °C (lit.)
Punto di fusione
−29 °C (lit.)
Densità
0.925 g/mL at 20 °C (lit.)
Gruppo funzionale
carboxylic acid
Stringa SMILE
CC(C)CC(O)=O
InChI
1S/C5H10O2/c1-4(2)3-5(6)7/h4H,3H2,1-2H3,(H,6,7)
GWYFCOCPABKNJV-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Eye Dam. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
176.0 °F - Pensky-Martens closed cup
Punto d’infiammabilità (°C)
80 °C - Pensky-Martens closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Inborn errors of metabolism are caused by changes in specific enzymatic reactions and hundreds of different such alterations, which affect about 1 of every 5000 newborns, have been characterized.
Separation of Propionic acid; Acetic acid; Heptanoic acid; Isobutyric acid; Valeric acid; Isocaproic acid; Butyric acid; Isovaleric acid
Separation of Methyl oleate; Caprylic acid; Heptanoic acid; Methyl decanoate; Methyl dodecanoate; Myristic acid; Methyl palmitate; Methyl palmitoleate; Methyl stearate; Methyl linoleate; Methyl linolenate; Acetic acid; Arachidic acid; Behenic acid; Propionic acid; Isobutyric acid; Valeric acid; Isovaleric acid; Isocaproic acid; Butyric acid
Today, diverse studies report the benefits of probiotics, such as inhibitory effects on pathogens, aid in the management or prevention of chronic intestinal inflammatory diseases or atopic syndromes, and support to the immune system. Potential beneficial applications abound, researchers continue to evaluate the effictiveness and clarify the mechanisms of action of probiotics.
Protocolli
In this study, SPME was used for the analysis of free fatty acids in Parmesan cheese using a 65 μm Carbowax/divinylbenzene (DVB) SPME fiber. Headspace extraction of the cheese sample was conducted at 65 °C for 15 minutes and analyzed by GC with FID detection. SPME is ideal for analyzing the volatiles associated with solid food samples. The phase chemistry of the Nukol GC column provides excellent peak shape of acidic compounds.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.