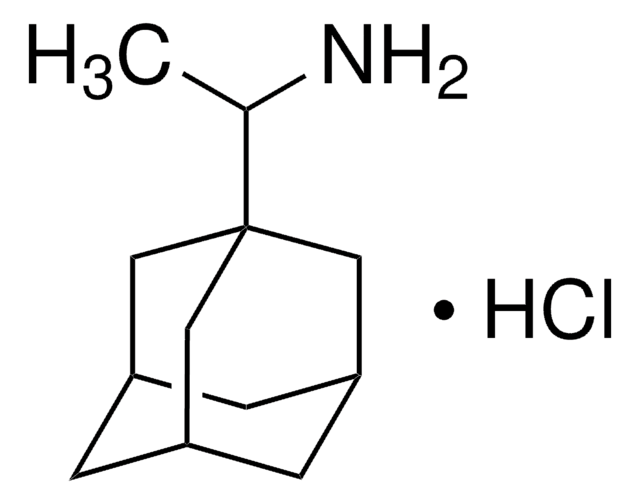
推荐产品
等級
pharmaceutical primary standard
API 家族
zanamivir
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
2-8°C
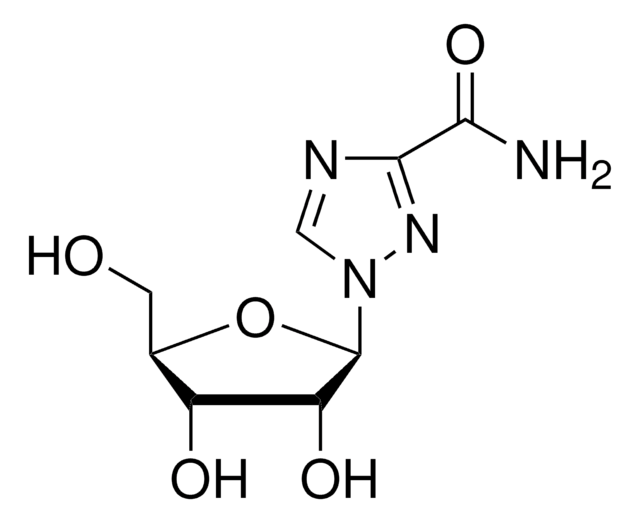
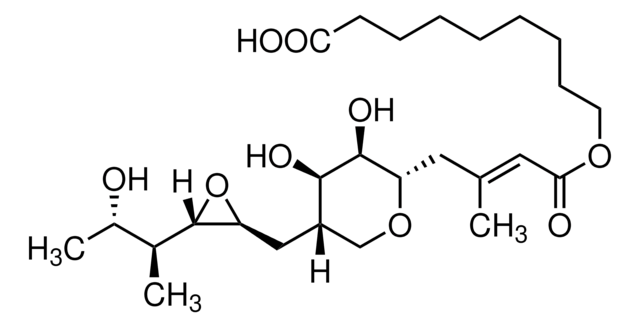
SMILES 字串
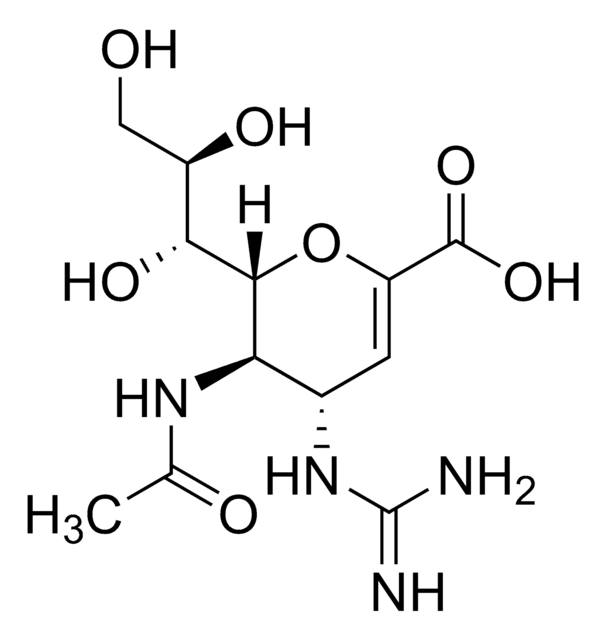
CC(=O)N[C@@H]1[C@@H](NC(N)=N)C=C(O[C@H]1[C@H](O)[C@H](O)CO)C(O)=O
InChI
1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1
InChI 密鑰
ARAIBEBZBOPLMB-UFGQHTETSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Zanamivir USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
生化/生理作用
Zanamivir is an influenza viral neuraminidase inhibitor.
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Susan Barrett et al.
Antiviral research, 108, 30-35 (2014-05-24)
The influenza virus neuraminidase inhibitors are normally slow binding inhibitors, but many mutations leading to resistance, also result in the loss of the slow binding phenotype. Mutations can also affect the rate of dissociation of the inhibitors from the neuraminidase
Kristin A Gabor et al.
Disease models & mechanisms, 7(11), 1227-1237 (2014-09-06)
Seasonal influenza virus infections cause annual epidemics and sporadic pandemics. These present a global health concern, resulting in substantial morbidity, mortality and economic burdens. Prevention and treatment of influenza illness is difficult due to the high mutation rate of the
Alireza Eshaghi et al.
Antimicrobial agents and chemotherapy, 58(12), 7188-7197 (2014-09-24)
Immunocompromised patients are predisposed to infections caused by influenza virus. Influenza virus may produce considerable morbidity, including protracted illness and prolonged viral shedding in these patients, thus prompting higher doses and prolonged courses of antiviral therapy. This approach may promote
Xiaoli Xiong et al.
Virology, 456-457, 179-187 (2014-06-04)
Mutant H5N1 influenza viruses have been isolated from humans that have increased human receptor avidity. We have compared the receptor binding properties of these mutants with those of wild-type viruses, and determined the structures of their haemagglutinins in complex with
Ken Watanabe et al.
Archives of medical research, 45(5), 359-365 (2014-06-01)
Influenza viruses are a serious threat to human health and cause thousands of deaths annually. Thus, there is an urgent requirement for the development of novel anti-influenza virus drugs. Therefore, the aim of this study was to evaluate the anti-influenza
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门