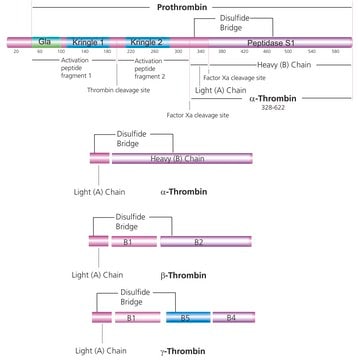
推荐产品
化驗
≥98% (HPLC)
形狀
solid
顏色
white
溶解度
DMSO: ≥40 mg/mL
H2O: insoluble
起源
Bayer
儲存溫度
2-8°C
SMILES 字串
OC(=O)CCn1c2CC[C@H](Cc2c3ccccc13)NS(=O)(=O)c4ccc(F)cc4
InChI
1S/C21H21FN2O4S/c22-14-5-8-16(9-6-14)29(27,28)23-15-7-10-20-18(13-15)17-3-1-2-4-19(17)24(20)12-11-21(25)26/h1-6,8-9,15,23H,7,10-13H2,(H,25,26)/t15-/m1/s1
InChI 密鑰
LDXDSHIEDAPSSA-OAHLLOKOSA-N
生化/生理作用
Ramatroban aids in the reduction of myocardial ischemia-reperfusion injury, lipopolysaccharide-induced shock, and vagal neuroeffector transmission in the tracheal smooth muscle of experimental models. In addition, it also suppresses allergen and IgE antibody-mediated skin and nasal reactions and eosinophilia in animal models with asthma.
Ramatroban is used for the treatment of allergic rhinitis as an antagonist of the thromboxane receptor. It is also an antagonist of the DP2 receptor with IC50 values of 100-170 nM. It is more potent at the DP2 receptor than the TP receptor by 4-5 fold. It is therefore a useful tool for the elucidation of PGD2/DP2 function in eosinophils, basophils, and other cells of the TH2 cell-type.
特點和優勢
This compound was developed by Bayer. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Hiromi Sugimoto et al.
European journal of pharmacology, 524(1-3), 30-37 (2005-11-01)
We previously showed that ramatroban (Baynastrade mark), a thromboxane A(2) (TxA(2)) antagonist, had inhibited prostaglandin D(2) (PGD(2))-stimulated human eosinophil migration mediated through activation of chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2). However, detailed pharmacological characterization of its inhibitory activity
Toshiaki Ishizuka et al.
Cardiovascular drug reviews, 22(2), 71-90 (2004-06-05)
It is known that thromboxane A2 (TXA2) contributes to various diseases such as bronchial asthma, ischemic heart disease, cerebrovascular disorders and allergic rhinitis. A number of TXA2 synthase inhibitors and TXA2 receptor (TP receptor) antagonists have been developed to treat
Stéphanie Rolin et al.
European journal of pharmacology, 533(1-3), 89-100 (2006-02-07)
COPD (Chronic Obstructive Pulmonary Disease) and bronchial asthma are two severe lung diseases which represent a major problem of world public health. Leukotrienes and prostanoids play an important role in the pathogenesis of pulmonary diseases. Prostanoids: prostaglandins (PGs) and thromboxane
Petra Schratl et al.
European journal of immunology, 36(9), 2401-2409 (2006-08-15)
Several chemoattractants can regulate the recruitment of eosinophils to sites of inflammation, but the hierarchy among them is unknown. We observed here that eosinophil chemotaxis towards eotaxin or 5-oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE) was amplified up to sixfold in the presence of
Eduardo Busto et al.
The Journal of organic chemistry, 77(10), 4842-4848 (2012-04-21)
A chemoenzymatic asymmetric route for the preparation of enantiopure (R)-ramatroban has been developed for the first time. The action of lipases and oxidoreductases has been independently studied, and both were found as excellent biocatalysts for the production of adequate chiral
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门