所有图片(2)
About This Item
经验公式(希尔记法):
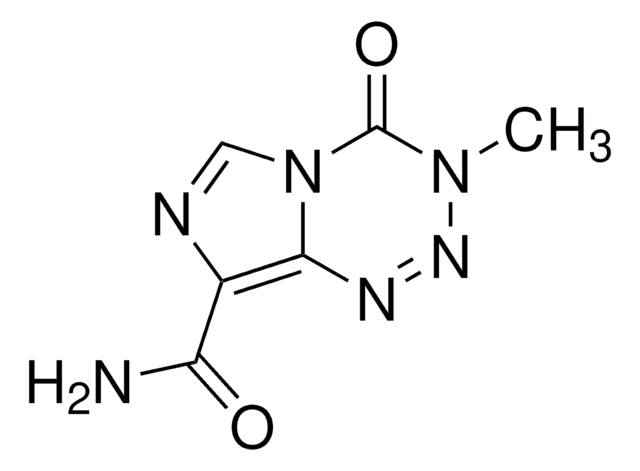
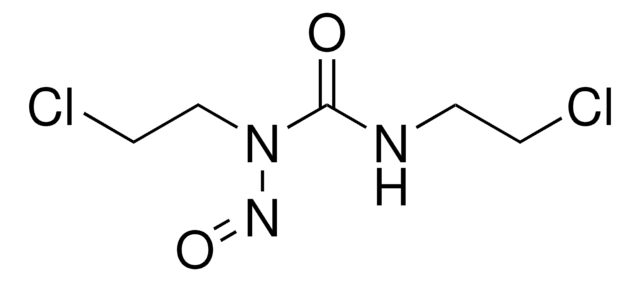
C9H16ClN3O2
CAS号:
分子量:
233.70
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.25
推荐产品
生化/生理作用
Antineoplastic agent with cellular DNA effects. Lomustine induces p53 expression in A2870 cells.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Carc. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Late and prolonged pseudoprogression in glioblastoma after treatment with lomustine and temozolomide.
Moritz Stuplich et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 30(21), e180-e183 (2012-06-13)
Roger J Packer et al.
Neuro-oncology, 15(1), 97-103 (2012-10-27)
The purpose of the trial was to determine the survival and incidence of secondary tumors in children with medulloblastoma receiving radiotherapy plus chemotherapy. Three hundred seventy-nine eligible patients with nondisseminated medulloblastoma between the ages of 3 and 21 years were
Autumn L Dutelle et al.
Journal of feline medicine and surgery, 14(10), 694-700 (2012-05-12)
This retrospective study evaluated the use of lomustine as a rescue agent for 39 cases of resistant feline lymphoma. Parameters assessed included lymphocyte cell size, number of previous chemotherapy drugs and number of previous chemotherapy protocols received, time from lymphoma
Thierry Gorlia et al.
European journal of cancer (Oxford, England : 1990), 49(16), 3477-3485 (2013-07-31)
The prognosis of patients with anaplastic oligodendrogliomas (AOD) and oligoastrocytomas (AOA) is variable. Biomarkers might be helpful to identify more homogeneous disease subtypes and improve therapeutic index. The aim of this study is to develop new clinical, pathological and molecular
Enrico Franceschi et al.
Neuro-oncology, 14(12), 1503-1510 (2012-10-24)
The treatment of patients with recurrent glioblastoma remains a major oncologic problem, with median survival after progression of 7-9 months. To determine the maximum tolerated dose and dose-limiting toxicity (DLT), the combination of dasatinib and cyclonexyl-chloroethyl-nitrosourea (CCNU) was investigated in
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门