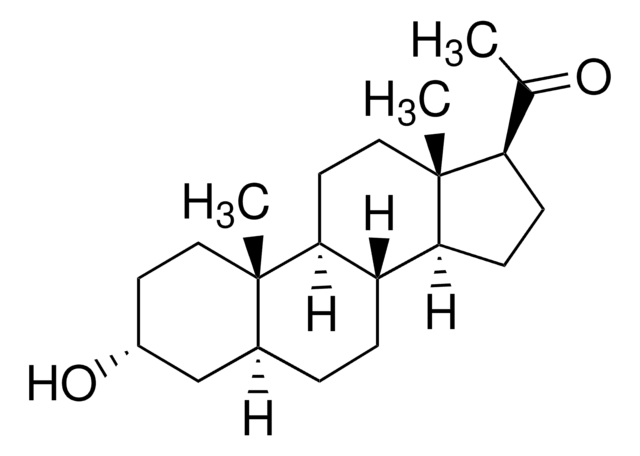
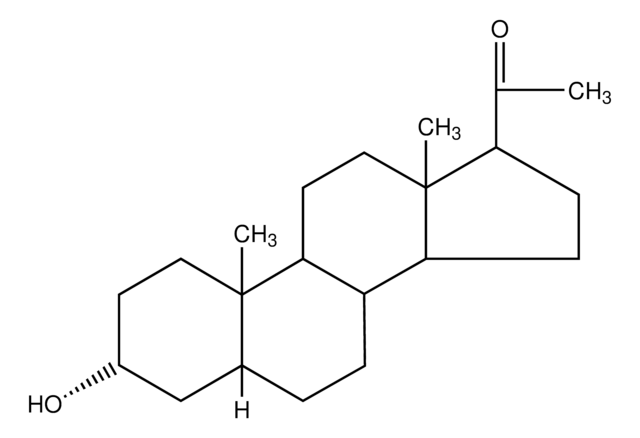
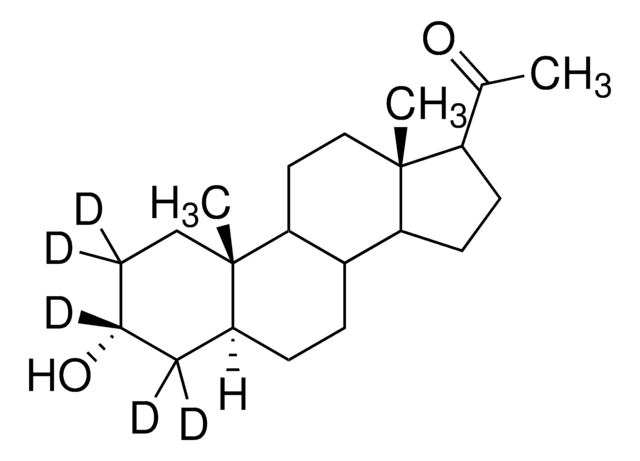
About This Item
推荐产品
品質等級
化驗
≥98% (HPLC)
形狀
solid
顏色
white
溶解度
DMSO: ≥2 mg/mL
H2O: insoluble
儲存溫度
2-8°C
SMILES 字串
[H][C@@]12CC[C@@]3([H])[C@]4([H])CC[C@H](C(C)=O)[C@@]4(C)CC[C@]3([H])[C@@]1(C)CC[C@@](C)(O)C2
InChI
1S/C22H36O2/c1-14(23)17-7-8-18-16-6-5-15-13-20(2,24)11-12-21(15,3)19(16)9-10-22(17,18)4/h15-19,24H,5-13H2,1-4H3/t15-,16-,17+,18-,19-,20+,21-,22+/m0/s1
InChI 密鑰
PGTVWKLGGCQMBR-FLBATMFCSA-N
基因資訊
human ... GABRA1(2554) , GABRA2(2555) , GABRA3(2556) , GABRA4(2557) , GABRA5(2558) , GABRA6(2559) , GABRB1(2560) , GABRB2(2561) , GABRB3(2562) , GABRD(2563) , GABRE(2564) , GABRG1(2565) , GABRG2(2566) , GABRG3(2567) , GABRP(2568) , GABRQ(55879)
mouse ... Gabrg2(14406)
生化/生理作用
特點和優勢
品質
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
商品
We offer many products related to GABAA receptors for your research needs.
We offer many products related to GABAA receptors for your research needs.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门