SLFG025LS
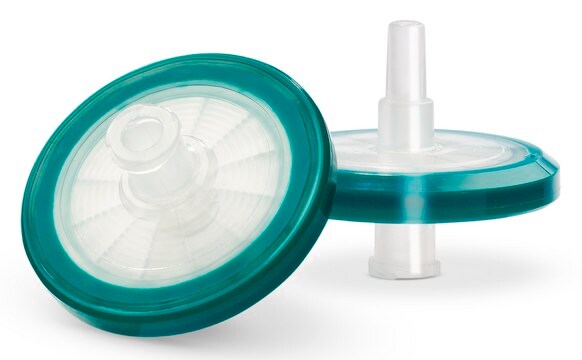
Millex®-FG 过滤装置 (无菌)
pore size 0.2 μm, diam. 25 mm, PTFE membrane, hydrophobic, medical device
别名:
Millex-FG 0.20µm Filter Unit or Vent Filter, Millex-FG Filter Unit, disposable syringe filter, sterile syringe filter, syringe filter
登录查看公司和协议定价
所有图片(1)
About This Item
分類程式碼代碼:
41104922
eCl@ss:
32031690
NACRES:
NB.22
推荐产品
材料
PTFE membrane
PVC housing
品質等級
無菌
sterile; ethylene oxide treated
產品線
Millex®
特點
CE compliant
holdup volume≤ 0.1 mL
hydrophobic
FDA registered
medical device
製造商/商標名
Millex®
參數
45 °C max. temp.
5.2 bar max. inlet pressure (75 psi)
技術
gas filtration: suitable
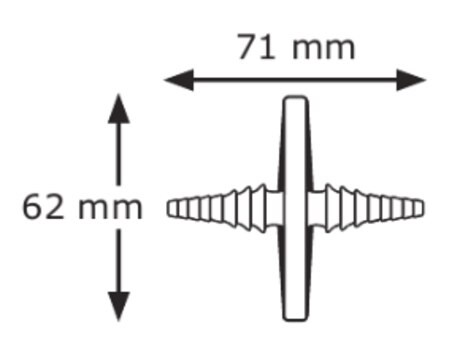
直徑
25 mm
過濾面積
4 cm2
housing diam.
29 mm
inlet to outlet H
25 mm
容積
100 mL
基質
Fluoropore®
孔徑
0.2 μm pore size
接頭
female Luer-Lok® inlet
male Luer outlet slip
運輸包裝
ambient
一般說明
Millex®-FG 过滤器(无菌)是一种医疗设备,可用作针头过滤器,用于患者直接护理和药房加药配液应用中不兼容标准薄膜过滤器的气体或小体积溶液的灭菌。
特點和優勢
- 双向支持的气体或液体灭菌过滤器
- 临床获益包括去除气态溶液中大于滤膜额定孔径的微生物、颗粒和未溶解粉末,从而减少交叉污染
- 设备的疏水膜阻止水溶液过滤通过
- 仅限一次性使用
- 无热原和无毒
- 经 CE 认证,并在 FDA 注册
其他說明
设备仅可用于医疗和/或实验室专业用途。
法律資訊
Fluoropore is a registered trademark of Merck KGaA, Darmstadt, Germany
Luer-Lok is a registered trademark of Becton-Dickinson & Co.
Millex is a registered trademark of Merck KGaA, Darmstadt, Germany
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门