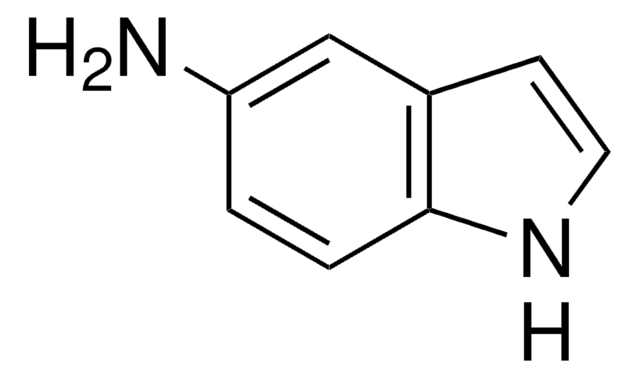
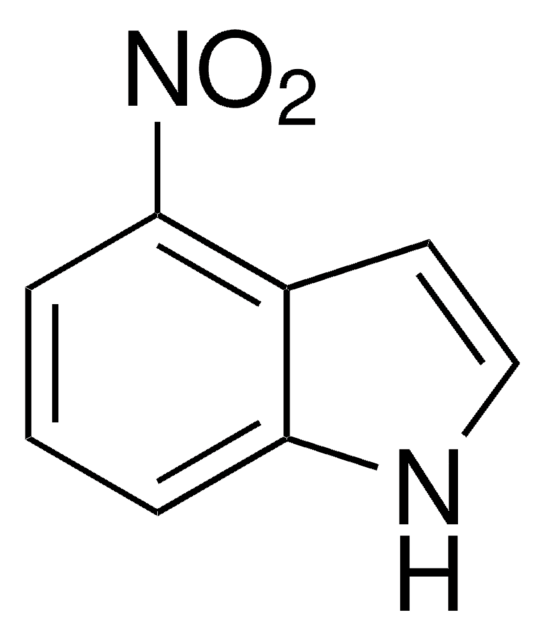
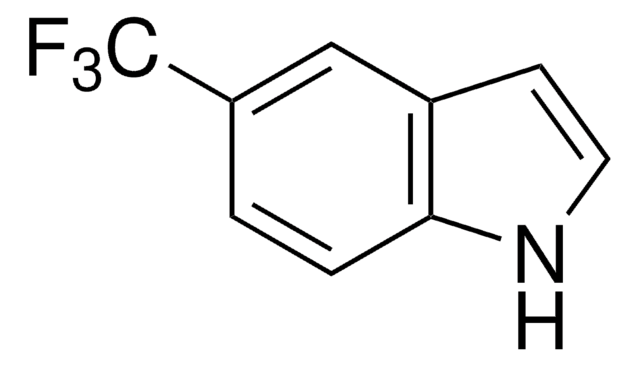
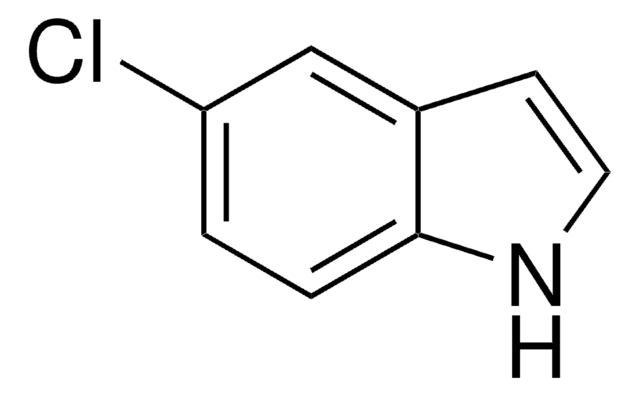
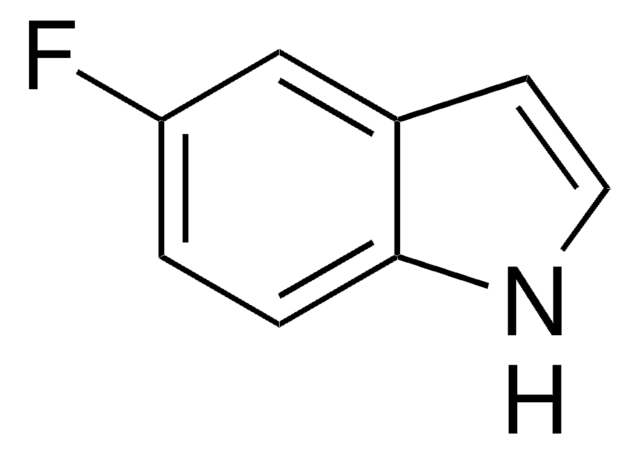
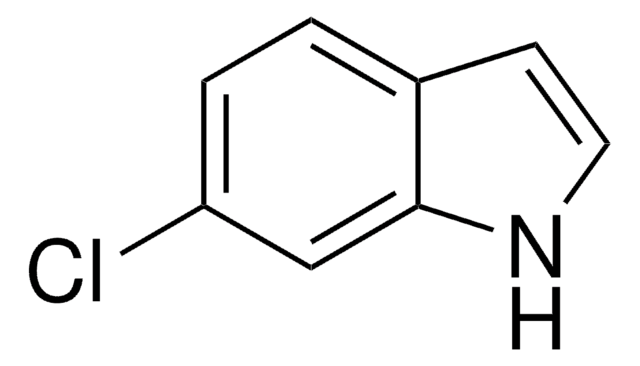
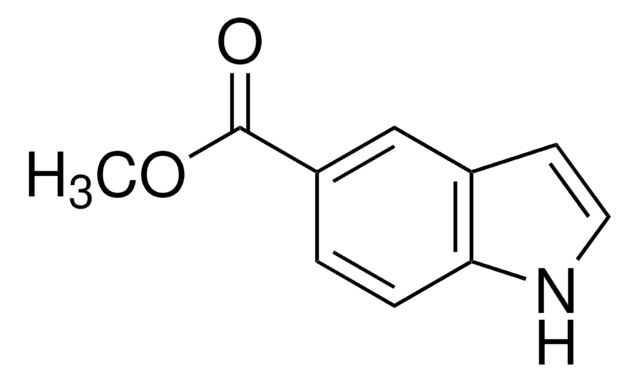
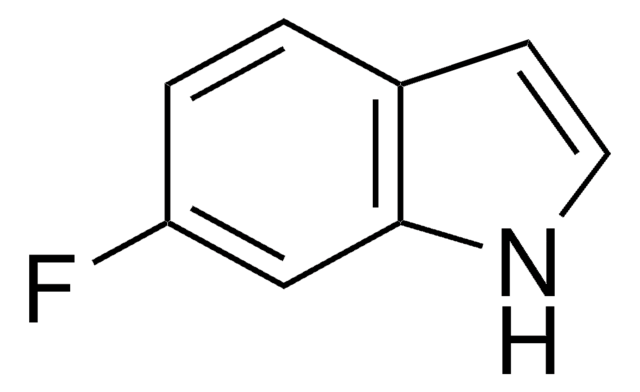
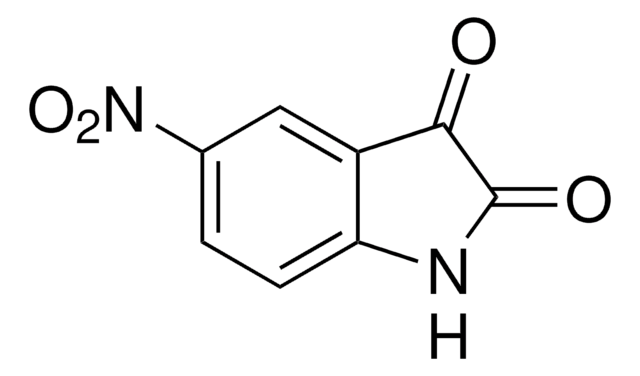
推荐产品
品質等級
化驗
98%
形狀
powder
mp
140-142 °C (lit.)
SMILES 字串
[O-][N+](=O)c1ccc2[nH]ccc2c1
InChI
1S/C8H6N2O2/c11-10(12)7-1-2-8-6(5-7)3-4-9-8/h1-5,9H
InChI 密鑰
OZFPSOBLQZPIAV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
作为以下制备反应的反应物:
- 具有药物活性的 2-氧代-1-吡咯烷类似物
- 色氨酸双加氧酶抑制剂吡啶基-乙烯基-吲哚类化合物可作为潜在的抗癌免疫调节剂
- 蛋白激酶抑制剂和抗增殖剂
- 代谢型谷氨酸受体4(mGlu4)的正变构调节剂。
- 抗真菌剂
- 大麻素1型受体(CB1)拮抗剂
- 潜在抗癌剂
- 潜在的抗血管药
- 选择性抗白血病药
- 抗人类免疫缺陷病毒亚型1(HIV-1)的药物
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Dam. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
其他客户在看
José Gallego et al.
Nucleic acids research, 35(9), 2904-2912 (2007-04-18)
Universal bases hybridize with all other natural DNA or RNA bases, and have applications in PCR and sequencing. We have analysed by nuclear magnetic resonance spectroscopy the structure and dynamics of three DNA oligonucleotides containing the universal base analogues 5-nitroindole
H Challa et al.
Organic letters, 1(10), 1639-1641 (2000-06-03)
[formula: see text] The syntheses of PNA oligomers containing potential ambiguous nucleobase analogues, namely 3-nitropyrrole and 5-nitroindole, have been accomplished. Hybridization properties of these PNAs with complementary oligodeoxynucleotides were evaluated by thermal denaturation experiments. Both novel residues exhibited little variation
P M Vallone et al.
Nucleic acids research, 27(17), 3589-3596 (1999-08-14)
Effects of the universal base 5-nitroindole on the thermodynamic stability of DNA hairpins having a 6 bp stem and four base loops were investigated by optical absorbance and differential scanning calorimetry techniques. Melting studies were conducted in buffer containing 115
Use of 5-nitroindole-2'-deoxyribose-5'-triphosphate for labelling and detection of oligonucleotides.
C L Smith et al.
Nucleosides & nucleotides, 17(1-3), 555-564 (1998-08-26)
The 5'-triphosphate of 5-nitroindole-2'-deoxyriboside has been shown to be a good substrate for terminal deoxynucleotidyl transferase (TdT). An antibody has been prepared for the detection of 5-nitroindole and has been used for the detection of 5-nitroindole tailed DNA both in
David Loakes et al.
Journal of the American Chemical Society, 131(41), 14827-14837 (2009-09-26)
Hydrophobic base analogues (HBAs) have shown great promise for the expansion of the chemical and coding potential of nucleic acids but are generally poor polymerase substrates. While extensive synthetic efforts have yielded examples of HBAs with favorable substrate properties, their
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门