推荐产品
品質等級
化驗
99%
光學活性
[α]20/D ≥+98°, c = 1 in ethanol
mp
109-111 °C (lit.)
SMILES 字串
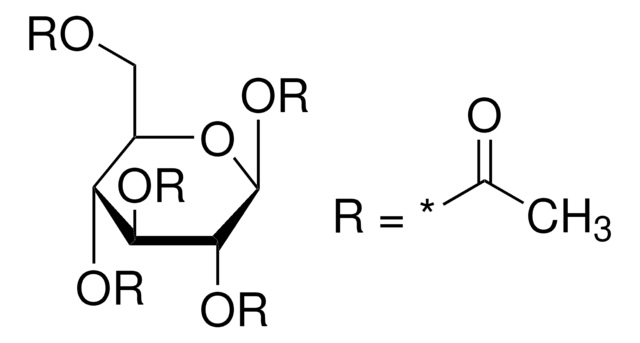
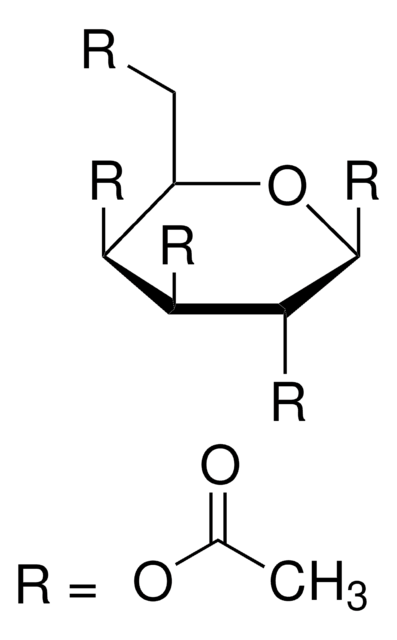
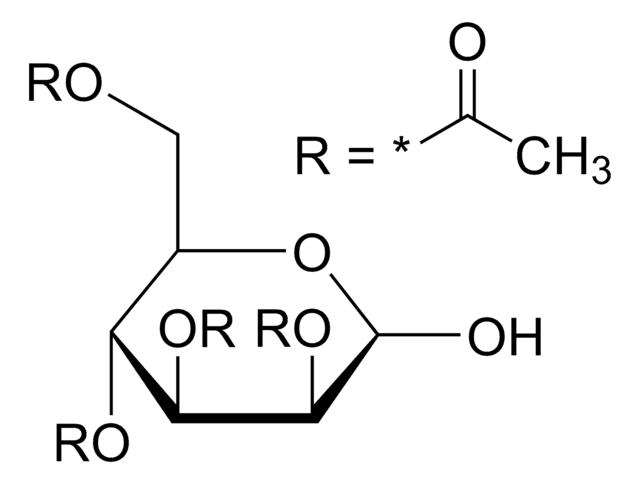
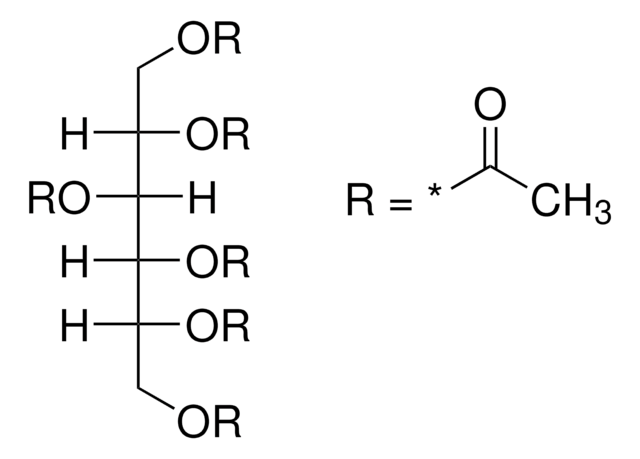
CC(=O)OC[C@H]1O[C@H](OC(C)=O)[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@@H]1OC(C)=O
InChI
1S/C16H22O11/c1-7(17)22-6-12-13(23-8(2)18)14(24-9(3)19)15(25-10(4)20)16(27-12)26-11(5)21/h12-16H,6H2,1-5H3/t12-,13-,14+,15-,16+/m1/s1
InChI 密鑰
LPTITAGPBXDDGR-LJIZCISZSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
α-D(+)-Glucose pentaacetate also known as 1,2,3,4,6-penta-O-acetyl-α-D-glucopyranose, is an acetylated sugar that has wide applications in organic synthesis.
應用
α-D(+)-Glucose pentaacetate is used as a model compound to study the stereochemistry of carbohydrates through spectroscopic techniques such as Vibrational circular dichroism (VCD). Additionally, it has been used as a standard in the analysis of monosaccharide and polysaccharide components by gas chromatography.
訊號詞
Warning
危險聲明
危險分類
Skin Sens. 1A
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
F Ponticelli et al.
Carbohydrate research, 330(4), 459-468 (2001-03-28)
We describe the synthesis of some 3-tert-butyl-4-hydroxyphenyl D-glycopyranosides by reaction of tert-butylhydroquinone with beta-D-pentaacetyl-glucose, beta-D-pentaacetyl-galactose, 2-acetamido- and 3,4,6-tri-O-acetyl-2-butanamido-2-deoxy-beta-D-glucopyranosyl chlorides as well as the formation of anomeric 3-tert-butyl-4-hydroxyphenyl 4,6-di-O-acetyl-2,3-dideoxy-D-erythro-hex-2-eno-pyranosides by reaction between tert-butylhydroquinone and 3,4,6-tri-O-acetyl-D-glucal. All compounds, except 3-tert-butyl-4-hydroxyphenyl alpha- and
W J Malaisse
Acta clinica Belgica, 57(2), 49-52 (2002-08-03)
The first report dealing with the use of monosaccharide esters as new tools in biomedicine was published in 1997 (1). This topic was first reviewed in 1998 (2). The major aim of the present report is to briefly evoke the
Shaji K Chacko et al.
Journal of applied physiology (Bethesda, Md. : 1985), 104(4), 944-951 (2008-01-12)
We report a new method to measure the fraction of glucose derived from gluconeogenesis using gas chromatography-mass spectrometry and positive chemical ionization. After ingestion of deuterium oxide by subjects, glucose derived from gluconeogenesis is labeled with deuterium. Our calculations of
W J Malaisse et al.
Archives of biochemistry and biophysics, 381(1), 61-66 (2000-10-06)
Hepatocytes from fed rats were incubated for 120 min in the presence of alpha-D-[1,2-13C]glucose pentaacetate (1.7 mM), both D-[1,2-13C]glucose (1.7 mM) and acetate (8.5 mM), alpha-D-glucose penta[2-13C]acetate (1.7 mM), or D-[1,2-13C]glucose (8.3 mM). The amounts of 13C-enriched L-lactate and D-glucose
H Jijakli et al.
Endocrine, 10(3), 219-224 (1999-09-14)
The metabolism of alpha-D-glucose pentaacetate and its positive insulinotropic action in isolated rat pancreatic islets are both unexpectedly resistant to D-mannoheptulose, as judged from experiments conducted over 90-120 min incubation. In the present study, the possible effects of the heptose
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门