推荐产品
形狀
powder or crystals
光學純度
ee: ≥99% (HPLC)
反應適用性
reagent type: ligand
環保替代產品特色
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
官能基
phosphine
環保替代類別
一般說明
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information.
應用
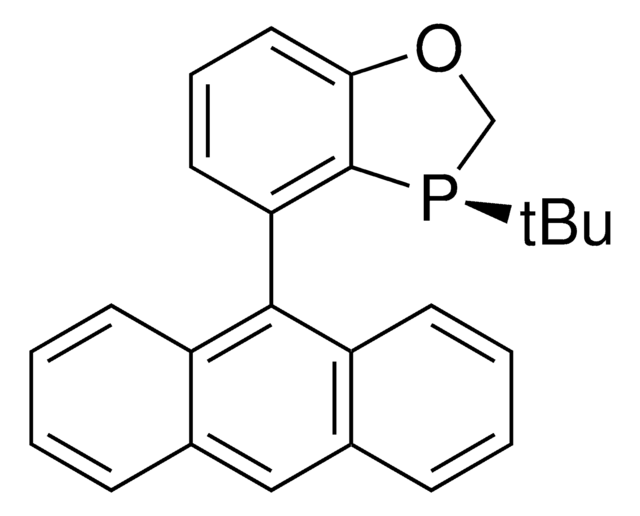
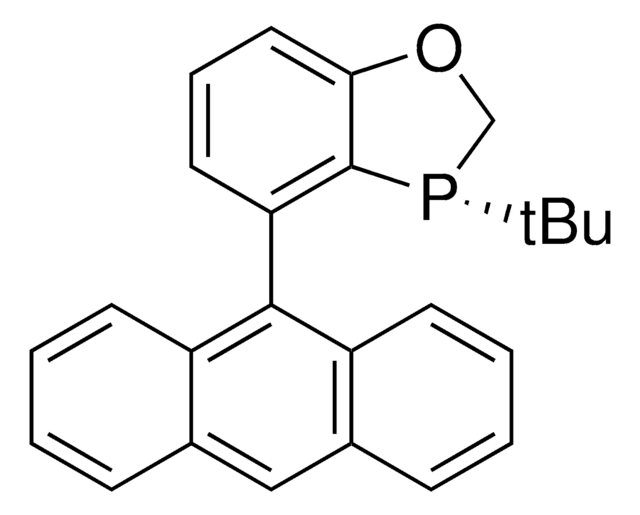
(2R,2R′,3R,3R′)-WingPhos is a P-chiral biphosphorus ligand for Rh-catalyzed asymmetric hydrogenations as well as Rh-catalyzed asymmetric addition of aryl boroxines to ketones.
Product can be used with our benchtop hydrogen generator, H-Genie Lite (Z744083)
Product can be used with our benchtop hydrogen generator, H-Genie Lite (Z744083)
法律資訊
Sold in collaboration with Zejun Pharmaceuticals
相關產品
产品编号
说明
价格
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Linwei Huang et al.
Angewandte Chemie (International ed. in English), 55(14), 4527-4531 (2016-03-05)
Highly enantioselective additions of arylboroxines to simple aryl ketones have been achieved for the first time with a Rh/(R,R,R,R)-WingPhos catalyst, thus providing a range of chiral diaryl alkyl carbinols with excellent ee values and yields. (R,R,R,R)-WingPhos has been proven to be
Jinbin Zhu et al.
Angewandte Chemie (International ed. in English), 58(45), 16119-16123 (2019-08-31)
Highly enantioselective rhodium-catalyzed addition of arylboroxines to N-unprotected ketimines is realized for the first time by employing chiral BIBOP-type ligands with a Rh loading as low as 1 mol %. A range of chiral α-trifluoromethyl-α,α-diaryl α-tertiary amines or 3-amino-3-aryloxindoles were formed with
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
![(R)-(-)-1-[(S)-2-二苯基磷]二茂铁乙基二环己基磷 ≥97%](/deepweb/assets/sigmaaldrich/product/structures/245/493/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0/640/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0.png)

![(R)-1-[(SP)-2-(二苯基膦)二茂铁基] 乙基二-叔丁基膦 ≥97%](/deepweb/assets/sigmaaldrich/product/structures/168/768/54a48841-6fe6-437a-81af-8c2e54117ef3/640/54a48841-6fe6-437a-81af-8c2e54117ef3.png)