推荐产品
化驗
≥95%
形狀
powder
光學純度
ee: ≥99% (HPLC)
反應適用性
reagent type: ligand
官能基
phosphine
應用
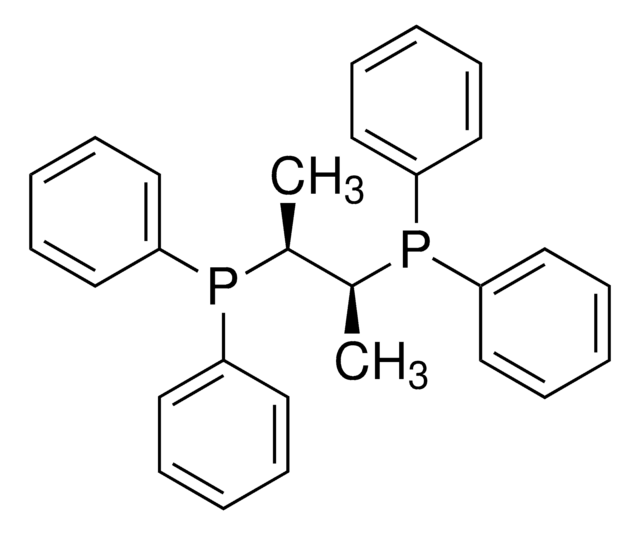
(2S,3S)-iPr-BIDIME is a P-chiral monophosphorus ligand used for the transition metal-catalyzed asymmetric Suzuki-Miyaura and hydroboration reactions.
法律資訊
Sold in collaboration with Zejun Pharmaceuticals
相關產品
产品编号
说明
价格
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Guangqing Xu et al.
Journal of the American Chemical Society, 136(2), 570-573 (2013-10-24)
Efficient asymmetric Suzuki-Miyaura coupling reactions are employed for the first time in total syntheses of chiral biaryl natural products korupensamine A and B in combination with an effective diastereoselective hydrogenation, allowing ultimately a concise and stereoselective synthesis of michellamine B.
Naifu Hu et al.
Journal of the American Chemical Society, 137(21), 6746-6749 (2015-05-06)
The rhodium-catalyzed asymmetric hydroboration of α-arylenamides with BI-DIME as the chiral ligand and (Bpin)2 as the reagent yields for the first time a series of α-amino tertiary boronic esters in good yields and excellent enantioselectivities (up to 99% ee).
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持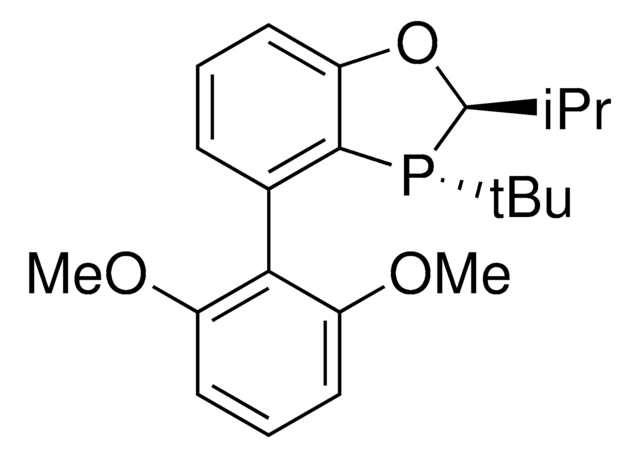
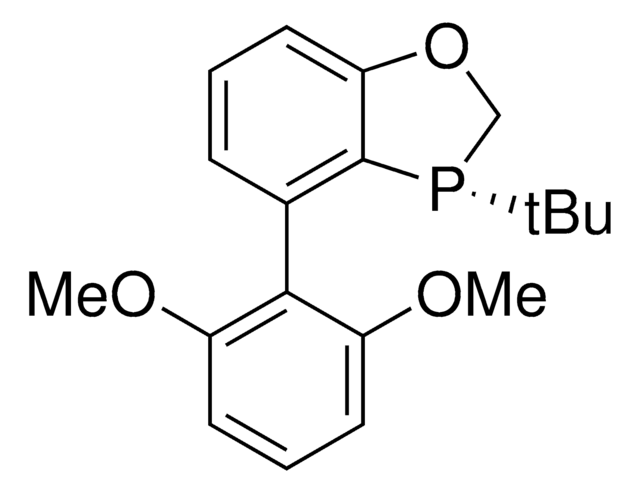
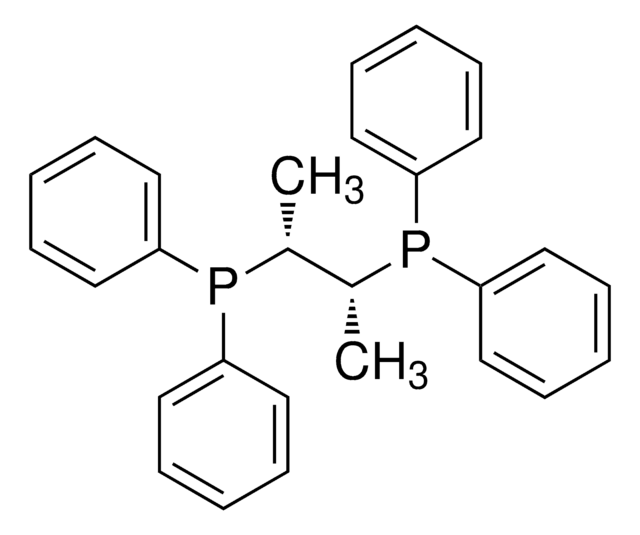
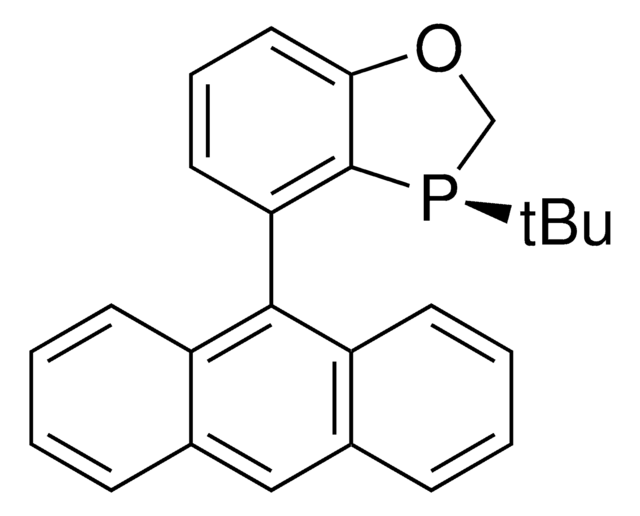
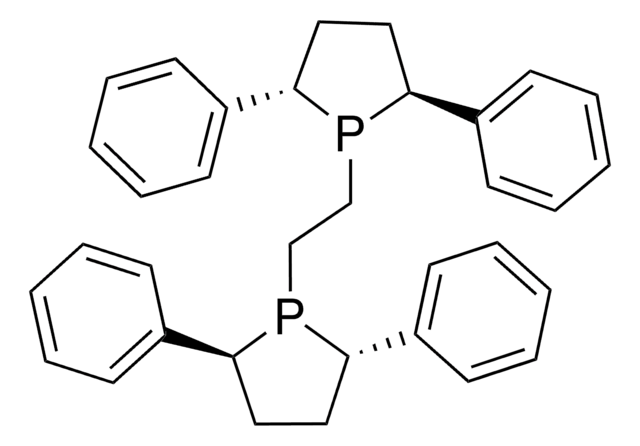
![(+)-1,2-双[(2S,5S)-2,5-二甲基磷]苯 kanata purity](/deepweb/assets/sigmaaldrich/product/structures/319/912/cec7b70f-bf7c-4a96-9f11-a73ae892e34c/640/cec7b70f-bf7c-4a96-9f11-a73ae892e34c.png)