901824
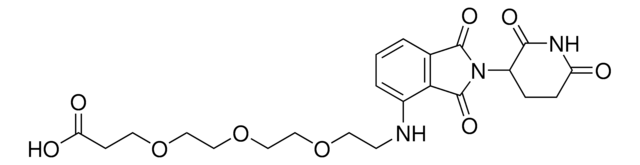
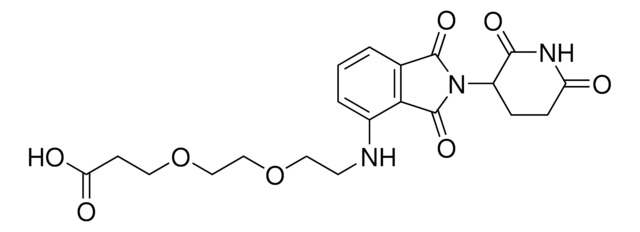
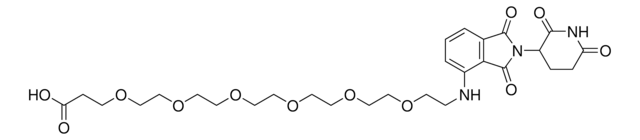
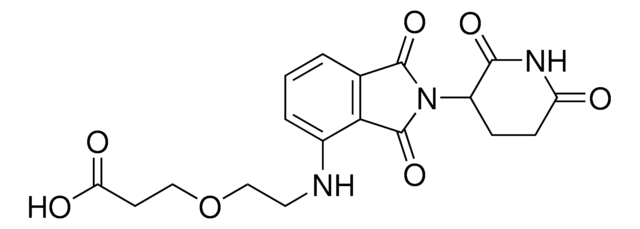
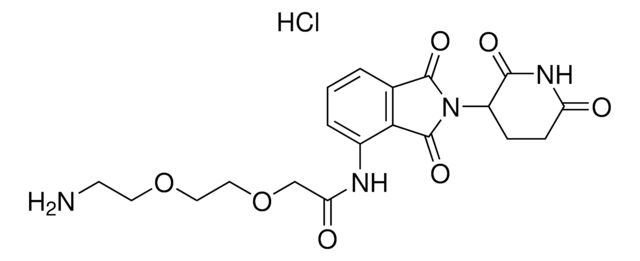
Pomalidomide-PEG4-CO2H
别名:
1-((2-(2,6-Dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)amino)-3,6,9,12-tetraoxapentadecan-15-oic acid, Crosslinker–E3 Ligase ligand conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
About This Item
推荐产品
ligand
pomalidomide
形狀
solid
反應適用性
reactivity: amine reactive
reagent type: ligand-linker conjugate
官能基
carboxylic acid
儲存溫度
2-8°C
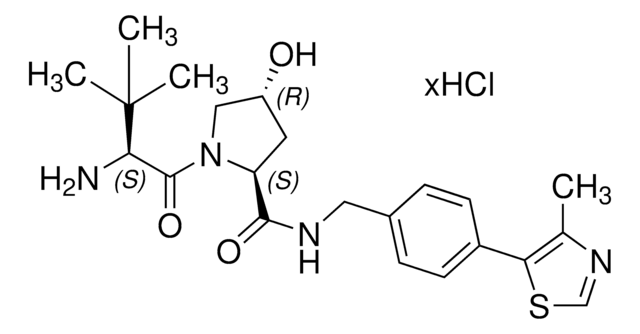
SMILES 字串
O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NCCOCCOCCOCCOCCC(O)=O)=O)NC1=O
應用
Automate your CRBN-PEG based PROTACs with Synple Automated Synthesis Platform (SYNPLE-SC002)
其他說明
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Targeted Protein Degradation by Small Molecules
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Development of Highly Potent and Selective Steroidal Inhibitors and Degraders of CDK8
法律資訊
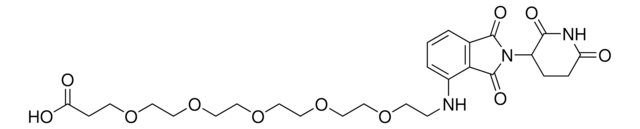
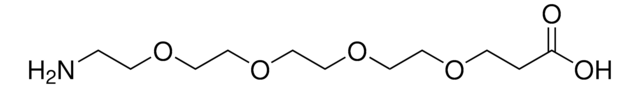
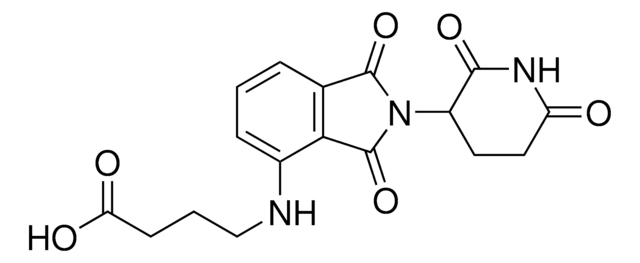
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
从最新的版本中选择一种:
其他客户在看
商品
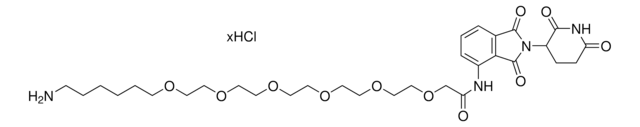
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Partial PROTACs are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门